Showing 1-15 of 78 results

Medical School News
Paul Picton, M.B., Ch.B., MRCP, FRCA, has been appointed interim chair of the Department of Anesthesiology in the Medical School, effective May 1. The provost has provided interim approval of his appointment, and it will be reported at the May Board of Regents meeting

Health Lab Podcast
The number of patients using cannabis for medical purposes has increased more than 600 percent since 2016.

Health Lab
Data on medical cannabis use found that enrollment in medical cannabis programs increased overall between 2016 and 2022, but enrollment in states where nonmedical use of cannabis became legal saw a decrease in enrollment

Medical School News
Four faculty and two staff members from the departments of Anesthesiology, Neurology, Radiology and Surgery, and the Office of Graduate Medical Education (GME), are recipients of GME Awards for 2024

Department News
University of Michigan Medical School Department of Anesthesiology residents present at the 2024 Midwest Anesthesia Residents Conference (MARC).

News Release
George A. Mashour, M.D., Ph.D., has been appointed senior associate dean for faculty and faculty development of the University of Michigan Medical School by the U-M Board of Regents, effective May 1, 2024.

Department News
A team led by Ashlee Holman, MD, recently completed its 1000th procedure under awake pediatric spinal anesthesia at C.S. Mott Children’s Hospital.

Department News
The University of Michigan Medical School Department of Anesthesiology's T32 research training program is one of 18 in the country focused on anesthesiology research.

Department News
Michigan Anesthesiology welcomes 27 newly matched residents to our residency program.

Health Lab Podcast
Therapies for pain conditions like fibromyalgia provide clues for helping those with long COVID.

Department News
The Office of the Vice President for Research has selected Rachel Bergmans, PhD, as one of 14 public engagement fellows.

Department News
A new study led by Michael Mathis, MD, is setting out to better understand variation in inotrope decision-making and outcomes across hospitals, as well as the barriers and facilitators to inotrope practice change. Mathis recently received National Institutes of Health funding through the National Heart, Lung and Blood Institute to support this research.

Department News
Nearly 80 nurse anesthetists, nurses, and nursing students from across the state gathered for a day of learning and networking during the Pediatric Nurse Anesthesia Conference
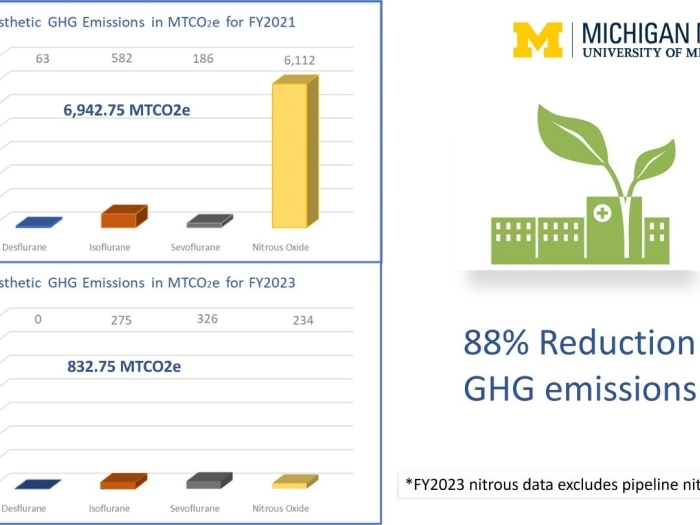
Department News
The Department of Anesthesiology’s Green Anesthesia Initiative (GAIA) has taken an ambitious approach to environmental sustainability resulting in an 88% reduction of inhaled anesthetic greenhouse gas emissions.

Health Lab
Therapies for pain conditions like fibromyalgia provide clues for helping those with long COVID-19