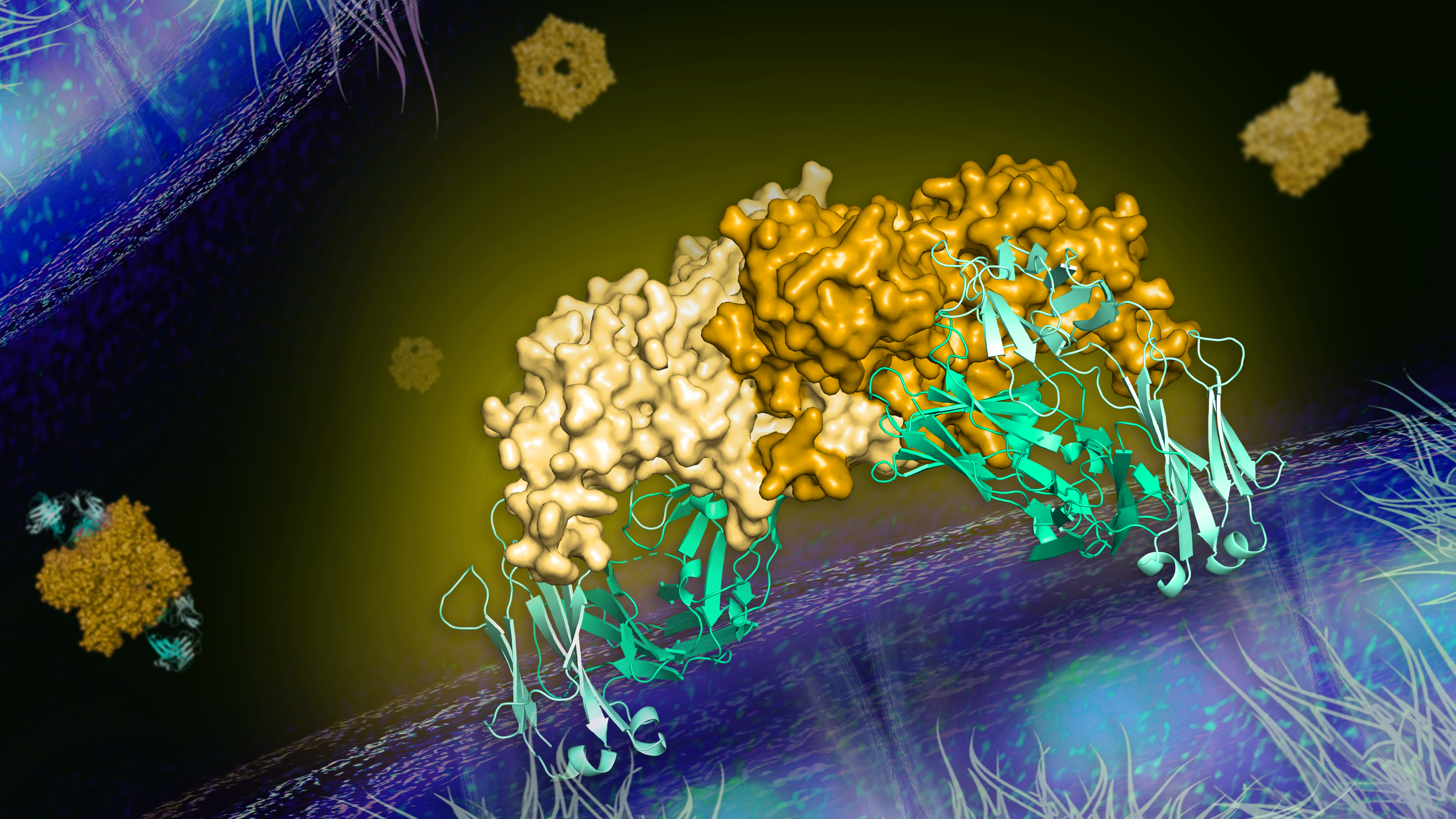
Flaviviruses are a group of RNA viruses that include the human pathogens dengue virus, Zika virus, and West Nile virus. Secreted nonstructural protein 1 (NS1) is a contributing factor to flavivirus pathogenesis. Despite demonstrated protection by NS1-specific antibodies against lethal flavivirus challenge, the structural and mechanistic basis has been unknown. In work published in Science, researchers in the laboratory of Janet Smith and their collaborators present three crystal structures of full-length dengue virus NS1 complexed with a flavivirus–cross-reactive, NS1-specific monoclonal antibody, 2B7. These structures reveal a protective mechanism by which two domains of NS1 are antagonized simultaneously. The NS1 wing domain mediates cell binding, whereas the β-ladder domain triggers downstream events, both of which are required for dengue, Zika, and West Nile virus NS1–mediated endothelial dysfunction. These observations provide a mechanistic explanation for 2B7 protection against NS1-induced pathology and demonstrate the potential of one antibody to treat infections by multiple flaviviruses.
Read the press release from UM Life Sciences Institute HERE.