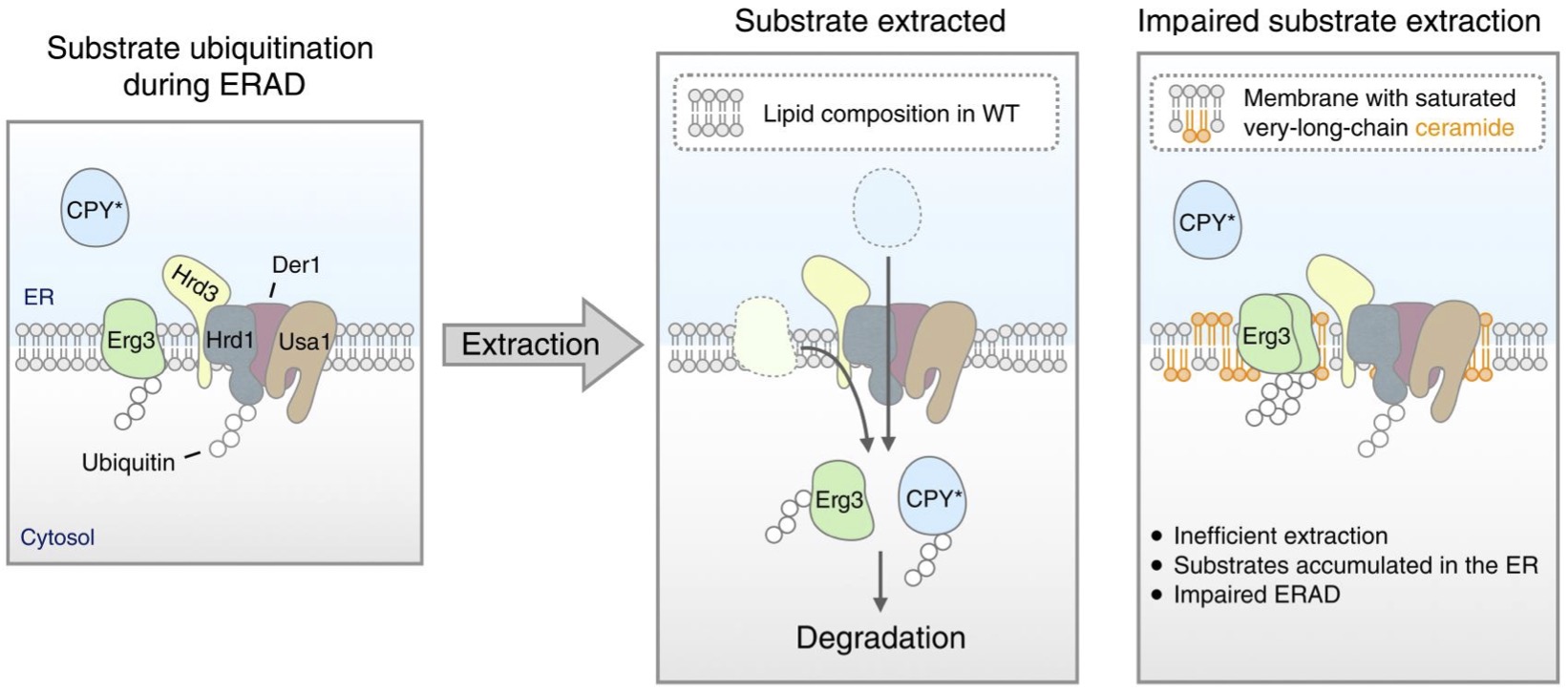
Misfolded proteins in the endoplasmic reticulum (ER) are removed through a process known as ER-associated degradation (ERAD). ERAD occurs through an integral membrane protein quality control system that recognizes substrates, retrotranslocates the substrates across the membrane, and ubiquitinates and extracts the substrates from the membrane for degradation at the cytosolic proteasome. In work recently published in Science Advances, researchers from the laboratory of Dr. Ryan Baldridge report that changes in the composition of the ER membrane can impair the function of the Hrd1-centric ERAD system. Using pharmacological and genetic manipulation to change the composition of the ER membrane, combined with unbiased lipidomic analysis, they find that elevation of specific very-long-chain ceramides restricts ERAD function, leading to substrate accumulation. This accumulation was not due to impaired ERAD complex assembly; under increased ceramide levels, the ERAD complex remained intact, and Hrd1 ubiquitination activity persisted. Rather, ubiquitinated ERAD substrates accumulated in the ER membrane, indicating that the extraction of substrates into the cytosol for degradation was disrupted under conditions with elevated ceramide levels. Their results reveal an intriguing interplay between lipid homeostasis and protein quality control and define a unique regulatory mechanism for ERAD activity.