What I’m Reading in APS Research, written by Ajay Tambralli, MD, is designed to provide relevant information about some of the latest research in APS and to help people better understand the research behind the publications. In this edition, Dr. Tambralli discusses three publications focusing on CAR T cells.
Using CAR T Cell Therapy for Rheumatic Diseases
For this newsletter, I am reviewing a concept called “CAR T cells”. These cells might eventually be used to treat a number of rheumatic diseases, including lupus and APS. One paper that I am discussing describes the use of CAR T cells in models of lupus and another is an abstract presented at the American College of Rheumatology (ACR) Annual Meeting last year that begins to bring this concept to APS.
Glossary of terms
- T cells: an important type of immune cell. T cells have many receptors on their surfaces, which serve different functions. Some of these receptors help the T cell do its various jobs, such as communicating with other immune cells to either amplify or reduce their functions. Other receptors are important because they recognize specific targets of the immune system - often bacteria, viruses, or other microbes. These latter receptors allow T cells to specifically focus their actions on the bad guys. These receptors can potentially be modified or engineered as discussed below.
- CAR T cells: chimeric antigen receptor T cells. These T cells have been engineered to have a new receptor that will target a human protein contributing to a disease (rather than a bacteria or virus). CAR T cell therapy has been used for several years in blood cancers like leukemia or lymphoma. The process of making CAR T cells starts by removing blood from an individual and then isolating T cells from the blood. This is followed by inserting an engineered gene into the T cells, which forces the cells to express the new receptor. The cells are then expanded in a lab. Finally, after enough T cells with the new receptor have been produced over several weeks, they are injected back into the same individual. Now, the T cells can go through the body looking for, and neutralizing, the target protein. There are several advantages to using CAR T cells including that they target proteins expressed only by specific cells, reach all organs and tissues in the body, and have long-lasting effects such that they only require one infusion. However, there are disadvantages to using CAR T cells, including something called cytokine release syndrome, which is when the immune system may become overactivated in a nonspecific way and cause a lot of collateral damage to the body.
- CD19: a protein that is specifically expressed on the surface of a different type of immune cell called a “B cell.” For most people, CD19-expressing B cells have beneficial functions because B cells make antibodies that help protect us from infections. However, in some individuals, a subgroup of B cells makes antibodies that attack the person’s own body, which then leads to autoimmune disease. Medicines or CAR T cells that target CD19 can help kill B cells.
Introduction
Lupus is an autoimmune disease that is sometimes present in people with APS. As with many autoimmune diseases, treatments for lupus do not work for everyone. And current treatments are not very targeted, which leaves lots of potential for side effects. In lupus, we know that B cells play a role in the disease by making autoantibodies. But, so far, medicines targeting B cells (such as rituximab) have not been very effective. There are many possible reasons for this, including that rituximab only targets some B cells, and may miss the “bad” B cells that live in places like the bone marrow that are difficult to reach. So, to overcome this, the authors of the first paper used CAR T cells engineered with a receptor that targets most B cells.
CAR T cells for lupus mice (paper #1)
What the authors did:
The authors first designed a new gene that would force T cells to express a new receptor for the CD19 protein that is present on B cells. They then delivered this gene into T cells to make the CAR T cells. Once they had made the CAR T cells, the authors used the cells to treat mice that are prone to developing severe lupus features.
What the authors found:
The mice that got the CAR T cell treatment had dramatically decreased lupus features as compared with mice that did not get the treatment. Importantly, the CAR T cell treatment also improved the lifespan of these lupus-prone mice. Notably, they found that the treatment was still active many months after the initial introduction of the CAR T cells, suggesting that this treatment had a long-lasting effect.
Despite the positive results above, there were some issues with the CAR T cell treatment, including that it did not always eradicate the B cells in every mouse (for reasons that were not completely understood). Another obvious point is that this was a study in mice and not humans, and we therefore don’t know what the long-term consequences of such a treatment might be in people with an autoimmune disease. However, we do know that cancer patients who have gotten CAR T cell treatments often do well.
CAR T cells for patients with lupus (paper #2)
A different group has now used similar CD19-targeting CAR T cell therapy in a few patients with lupus with promising results (2)! While only a small number of patients have been treated so far, this is an exciting potential treatment that could come to some specialty clinics in the near future.
Possible CAR T cells for APS (paper #3)
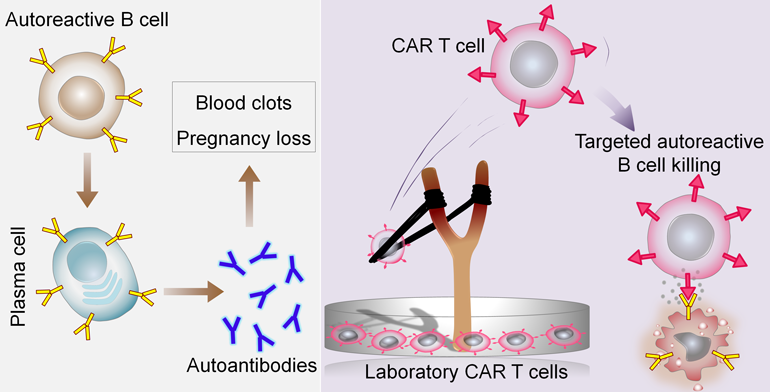
In APS, we understand that an autoantibody called anti-beta-2 glycoprotein I (anti-b2GPI) is one of the most problematic because it contributes to things like blood clots and pregnancy losses. We also know that B cells that produce these bad anti-b2GPI antibodies also display a version of the antibody itself on their surfaces. So, in an ideal world, we would target not all the B cells in the body (as many of them are good) but only those bad cells that make anti-b2GPI antibodies. This ideal approach would avoid hurting all B cells, allowing them to keep their beneficial functions.
An abstract presented at the ACR Annual Meeting shows the potential of such an approach. The authors engineered human T cells to recognize the anti-b2GPI antibody displayed on the surface of the bad B cells. In a cell culture dish, these engineered T cells were able to selectively bind to and eliminate only the B cells that were producing anti-b2GPI antibodies. This approach is still in the early stages and has not yet been tried in humans, but it could eventually result in a promising approach for treating APS.
Conclusions and next steps
Taken together, these efforts show the long road to discovering new treatments for a disease – starting with an idea, testing that idea in cell culture plate or other artificial systems in a lab, and finally translating it to individuals with a disease. It often takes years or decades to nurture this process into treatments. The hope is that the ongoing efforts discussed above will inspire more effective and more targeted treatments for individuals living with rheumatic diseases and, from our perspective, especially APS.
References
- Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, Ghani QU, Balazs L, Beranova-Giorgianni S, Giorgianni F, Kochenderfer JN, Marion T, Albritton LM, Radic M. Sci Transl Med. 2019 Mar 6;11(482):eaav1648. doi: 10.1126/scitranslmed.aav1648. PMID: 30842314; PMCID: PMC8201923.
- Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, Völkl S, Simon D, Kleyer A, Munoz L, Kretschmann S, Kharboutli S, Gary R, Reimann H, Rösler W, Uderhardt S, Bang H, Herrmann M, Ekici AB, Buettner C, Habenicht KM, Winkler TH, Krönke G, Schett G. Nat Med. 2022 Oct;28(10):2124-2132. doi: 10.1038/s41591-022-02017-5. Epub 2022 Sep 15. Erratum in: Nat Med. 2022 Nov 3;: PMID: 36109639.
- Chimeric Autoantigen-T Cell Receptor (CATCR)-T Cell Therapies to Selectively Target Autoreactive B Cells [abstract]. Mog B, Shaw E, Hwang M, Pearlman A, DiNapoli S, Paul S, Bettegowda C, Papadopoulos N, Gabelli S, Petri M, Rosen A, Zhou S, Kinzler K, Vogelstein B, Konig M. Arthritis Rheumatol. 2022; 74 (suppl 9).
Join Our Email List
If you are interested in receiving updates on our patient care and research efforts, please join our APS Program email list.



