What I’m Reading in APS Research, written by Ajay Tambralli, MD, is designed to provide relevant information about some of the latest research in APS and to help people better understand the research behind the publications. In this edition, Dr. Tambralli discusses an article published by our APS Program in 2017 and a recent publication in 2022 that provided new insights and updates to our work from 2017.
Targeting the Interaction Between Neutrophils and Endothelial Cells May Be a Future Therapy for APS
Glossary of Terms
- Neutrophils: the most common type of immune cell in circulation designed to be first responders to infection.
- Endothelial cells: these cells line the inside of blood vessels and normally help to keep blood moving smoothly.
- Gene: a segment of DNA that codes for a specific protein. Our DNA contains thousands of genes.
- PSGL-1 (P-selectin glycoprotein-1): a molecule that immune cells express on their surface to help stick to another protein called P-selectin that is found on other cells, particularly the endothelial cells. The interaction between these two helps one cell bind to the other.
- Transcription factor: a protein that binds to a specific part of DNA and helps convert a gene into another string of code called messenger RNA, which then goes on to be converted into a protein. Transcription factors help turn a gene on or off and are important in all facets of life.
- KLF2 (Krüppel-like factor 2): a transcription factor that has been found to reduce the activation of neutrophils. In addition, normal levels of KLF2 are beneficial to prevent blood clots.
- Nanoparticles: very small particles (a thousand times smaller than the width of a human hair) that can be made with different materials and are often coated with various compounds to target a specific molecule in the body.
Introduction
This month, I am reviewing the below two publications (one very recent) that studied the interactions between neutrophils and endothelial cells and in the process have advanced APS research.
(1) Activated Signature of Antiphospholipid Syndrome Neutrophils Reveals Potential Therapeutic Target
(2) A Targetable Pathway in Neutrophils Mitigates Both Arterial and Venous Thrombosis
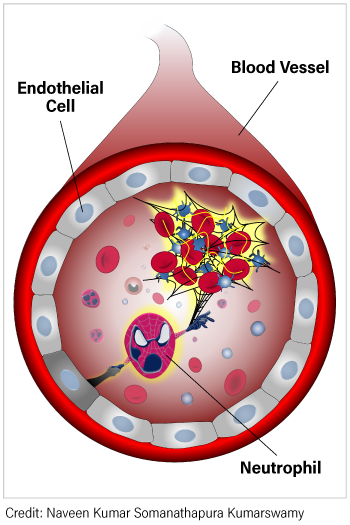
A lot of research effort in APS is going towards finding better treatments for this disease. As most people know, blood thinners like warfarin are the mainstay of treatment for APS, but they are often not enough to completely treat all aspects of the disease. One way to overcome this problem is to determine the causes of APS, and much of the work in this realm is trying to better understand how the immune system in APS is different. What our lab and others have uncovered is that neutrophils are hyperactive in APS. Efforts are now underway to figure out what makes them hyperactive, and how this hyperactivity affects their interactions with other cells. This is particularly important for how neutrophils interact with endothelial cells. Endothelial cells constantly patrol what’s happening in the bloodstream and normally act to prevent blood clots. Unfortunately, endothelial cells in APS do not always function normally and so may create an environment that makes it easier to form blood clots. So, it makes sense to determine how neutrophils and endothelial cells interact with each other in APS, which is exactly what was studied in the two papers that I will discuss in more detail below.
PSGL-1 on neutrophils is important in APS-associated blood clots
A few years ago, work by our research group (1) found that neutrophils from patients with APS have increased amounts of many genes that allow neutrophils to better stick to other cells. One of these genes codes for PSGL-1, and our next step was to determine whether PSGL-1 was important in forming blood clots. To do this, the gene for PSGL-1 was deleted in mice, which led to smaller blood clots when the mice received antibodies from patients with APS. Altogether, this research suggested that blocking PSGL-1, and thus blocking neutrophils from attaching to endothelial cells, might be a viable future therapy for APS.
Deleting KLF2 can lead to hyperactive neutrophils and increase blood clots
A group based out of Cleveland recently published an exciting paper (2) that confirmed some aspects of our earlier work. They started by looking at the effects of a health-promoting transcription factor called KLF2. The authors found that when mice were given antibodies from patients with APS, their neutrophils expressed less KLF2. Moreover, when the KLF2 gene was totally deleted, larger blood clots formed in a faster time frame, and more neutrophils were found at the site of the blood clotting.
KLF2 and PSGL-1 are linked in neutrophils
The next question they asked was what additional genes were altered in the neutrophils of the various mouse strains. To do this, they used a technique called RNA sequencing and found that when KLF2 was deleted, neutrophils expressed more genes that allow them to stick to other cells. They next used a system of very small channels that were coated with different adhesion proteins normally found on human cells. They discovered that in contrast to other adhesion factors, neutrophils with the KLF2 deletion attached more strongly to channels coated with P-selectin, which is the protein that PSGL-1 binds to! In fact, when given a drug that blocks PSGL-1, this effect was reversed. In short, when KLF-2 is lost (as likely happens in APS), neutrophils become stickier because of more PSGL-1.
Developing a better drug to target neutrophil and endothelial cell interactions
A big challenge in medicine is to reduce drug side effects, and one way to do this is to deliver medicines to only those places in the body where they are needed. In pursuit of such targeted delivery, the Cleveland group created nanoparticles that were coated with proteins that recognize clusters of PSGL-1, which are more likely to be found on APS neutrophils. This nanoparticle system was highly effective in neutralizing PSGL-1, which reduced both neutrophil attachment and blood clot size.
Conclusions and next steps
These papers show the process of attempting to discover the underlying mechanisms of APS, which in turn can help propel the discovery of better treatments. This process often takes years of effort and work in many different labs. The next steps for both research groups are likely to continue to better understand what exactly is happening in APS and to translate that knowledge back to patients to see if it can reveal better treatment options in the future.
References
- Knight JS, Meng H, Coit P, Yalavarthi S, Sule G, Gandhi AA, Grenn RC, Mazza LF, Ali RA, Renauer P, Wren JD, Bockenstedt PL, Wang H, Eitzman DT, Sawalha AH. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight. 2017 Sep 21;2(18):e93897. doi: 10.1172/jci.insight.93897. PMID: 28931754; PMCID: PMC5621930.
- Nayak L, Sweet DR, Thomas A, Lapping SD, Kalikasingh K, Madera A, Vinayachandran V, Padmanabhan R, Vasudevan NT, Myers JT, Huang AY, Schmaier A, Mackman N, Liao X, Maiseyeu A, Jain MK. A targetable pathway in neutrophils mitigates both arterial and venous thrombosis. Sci Transl Med. 2022 Aug 31;14(660):eabj7465. doi: 10.1126/scitranslmed.abj7465. Epub 2022 Aug 31. PMID: 36044595.
Join Our Email List
If you are interested in receiving updates on our patient care and research efforts, please join our APS Program email list.



