Synaptic Function & Dysfunction in the Auditory Nervous System

The synapse is the critical structure where neuronal information is transmitted, and presynaptic excitability is crucial for the reliable transmission of this information. However, it is very difficult to study presynaptic activity and vesicular neurotransmitter release directly at CNS nerve terminals because the sub-micron size of nerve terminals has precluded direct recordings. To study presynaptic properties directly, we take advantage of the calyx of Held, a large terminal in the central auditory system that allows direct recordings. These terminals provide a good model for synaptic transmission because they are a one-to-one synapse (i.e. one terminal to one postsynaptic neuron). Additionally, the role of synaptic transmission in auditory function and dysfunction is easily studied at this synapse. The long-term goal is to identify neurophysiological mechanisms underlying central auditory dysfunctions to improve preventive and therapeutic strategies for central auditory disorders caused by various peripheral hearing deficits (e.g. congenital deafness). Sound deprivation resulting from peripheral hearing deficits negatively impacts the central auditory circuit functions, even after peripheral sound sensitivity is restored, such as with hearing aids. Using patch-clamp recordings, advanced imaging techniques, and in vivo auditory function tests, we are studying synaptic activities and plasticity in auditory disorders during development or aging.
Neuron-Oligodendroglia Interaction
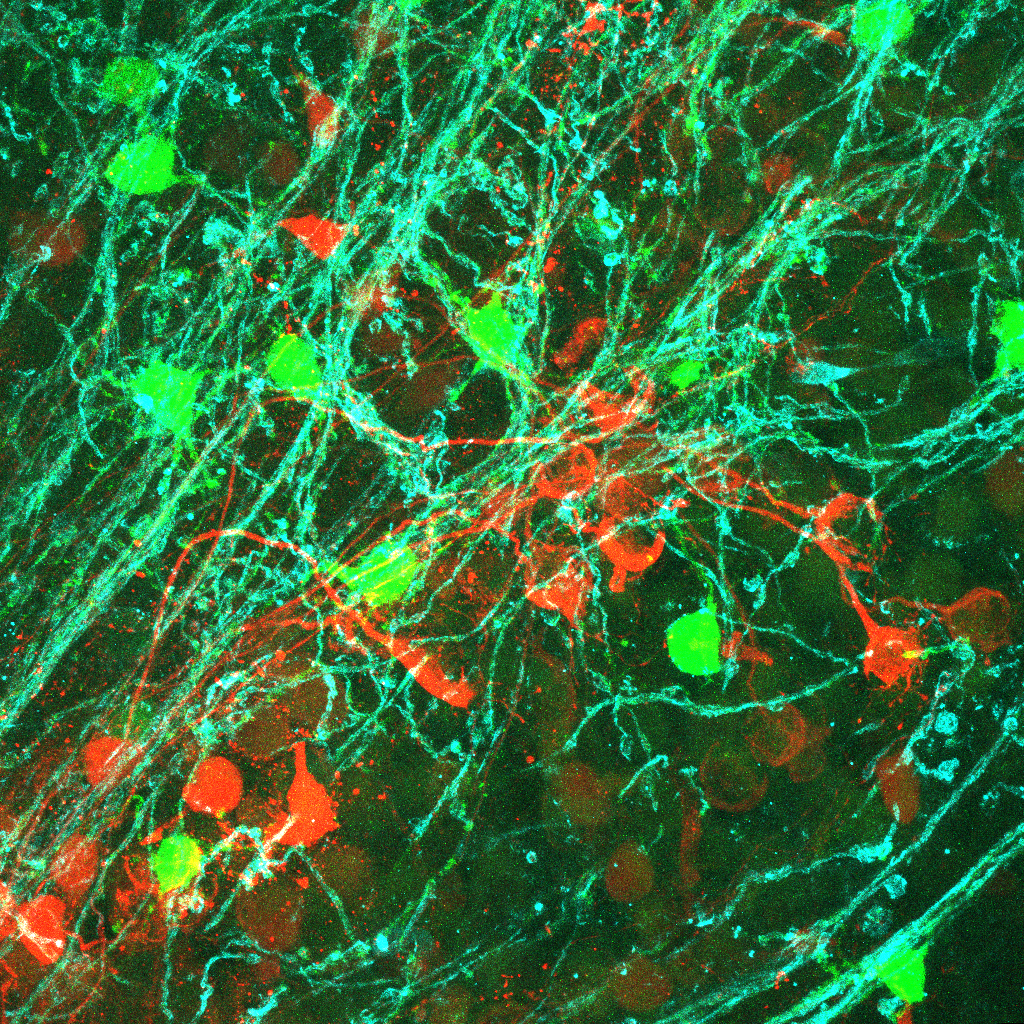
Auditory processing abnormalities (e.g. auditory hypersensitivity and reduced auditory spatial attention) are common and prominent features in neurodevelopmental disorders such as autism spectrum disorder (ASD). The long-term goal is to investigate the mechanisms how altered myelination and brain connectivity impair auditory processing in neurodevelopmental disorders. A better understanding of the contributions of the neural mechanisms underlying the auditory processing abnormalities to ASD will help guide better therapeutic strategies for individuals with ASD. Dynamic communication between neurons and myelin-forming oligodendrocytes (OLs) is pivotal in the strength and speed of neurotransmission by influencing myelination in the developing brain. Our recently published studies have created a foothold in understanding communication between neurons and OLs by defining OL excitability in the auditory brainstem. A subpopulation of OLs expresses glutamate receptors and voltage-gated Na+ and Ca2+ channels, which underlie OL depolarization, Na+ current-mediated spiking, and Ca2+ dynamics in OLs (Berret et al., 2017, Barron et al., 2019). A Ca2+ transient in OLs triggers release of brain-derived neurotropic factor (BDNF), important in synaptic strength and plasticity in the auditory brainstem (Jang et al., 2019). Currently, we are investigating how neurons and OLs communicate to control activity-dependent myelination in the mammalian brain.
Physiological and Genetic Profiles of Heterogeneous Oligodendrocytes
Spiking immature oligodendrocytes (OLs), referred to as spiking OLs, express voltage-activated Na+ channels (Nav) and K+ (Kv) channels, endowing a subpopulation of OLs with the ability to generate Nav-driven spikes. In this study, we investigate the molecular profile of spiking OLs, using single-cell transcriptomics paired with whole-cell patch-clamp recordings. Scn2a, which encodes the channel Nav1.2, is specifically expressed in spiking OLs in the brainstem and cerebellum, both in mice and in Olive baboons (Gould and Kim, 2021). Currently, we are characterizing transcriptional profiles of OL lineage cells and match them to their physiological properties by combining electrophysiological recordings with a state-of-the art technique, patch-seq transcriptomics. The patch-seq transcriptomics approach, which combines patch-clamp electrophysiology with post hoc morphological reconstruction, and genetic analysis by RNA-seq in single cells.

