Bacteria, viruses, parasites and fungi are all organisms that can interact with the human body, making us either healthy or sick. Our research spans from the cell to the organ, to the body and to the global population, investigating and impacting the very foundations of human health.

Here, graduate students and postdoctoral fellows work side-by-side with principal investigators, testing hypotheses of significant medical importance with the potential to translate into medical advancements. This groundbreaking laboratory training is supplemented with rigorous coursework, seminar series, journal clubs, and the presentation of research findings at national meetings.
1150 W. Medical Center Dr.
Ann Arbor, MI 48109-5632

We are pleased to share with you our 2022–23 Report with a special featured story on the history of the early women in M&I at the University of Michigan.

The A. Oveta Fuller Award is a career development award for emerging leaders in the areas of microbiology and immunology, infectious disease, or health disparities whose goals and accomplishments benefit society in the spirit of Dr. A. Oveta Fuller.
We are a diverse group of scientists, united by a passion for discovery, leadership, service and the sharing of knowledge to address global microbiology and immunology challenges. Our DEI activities, events, trainings, community-building efforts and data collection are valuable additions to the University’s broader strategic DEI plan.
Our department's motto is that 'We are an inclusive community whose passion for discovery, service, leadership and sharing of knowledge addresses global microbiology and immunology challenges.'"

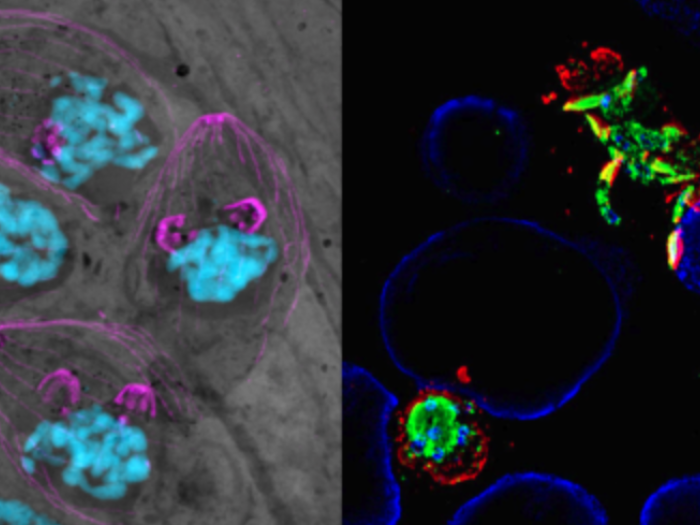
Attend the only regional conference that features cutting-edge basic science and translational research on the pathogenic mechanisms of fungal and parasitic diseases.



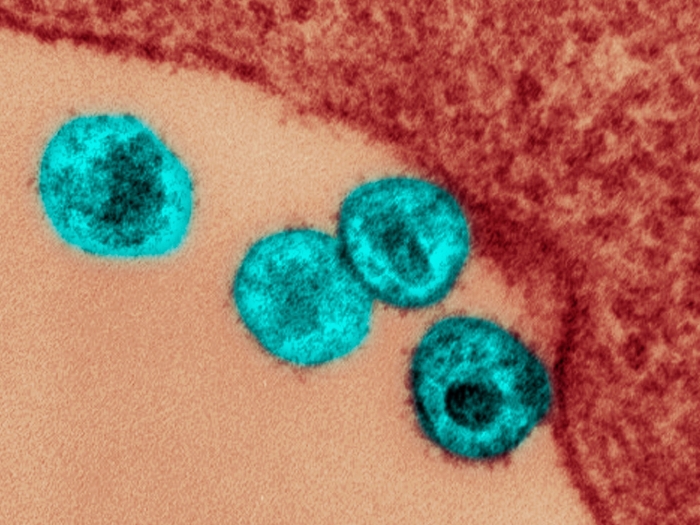



Resources and information for current learners and faculty.