Administrative Contact
Biography

Dr. Mukesh Nyati has over twenty years of experience in basic and translational oncology. He is a tenured Professor in the Department of Radiation Oncology at the University of Michigan, supervising junior faculty and post-doctoral fellows in the laboratory. His administrative responsibilities include organizing Cancer Biology Course that prepares Radiation Oncology residents for their board exams. Dr. Nyati actively participates in the peer review process for many journals and the grant review process for NIH, NCI, DOD, SBIR, and STTR. He served as a consultant for CTEP for clinical development of AT13387 and AZD9291.
Dr. Nyati is involved in three major type of research.
Basic Research
The main focus of his research is investigating the role of protein stability in cancer progression and treatment. Dr. Nyati has been studying EGFR, cMet and KRAS oncogenes and agents that either block their activity or induce selective degradation of these oncoproteins. His group also studies the roles of the EGFR protein independent of kinase activity in cell growth and DNA repair. He is also part of the efforts of the Protein Folding Disease Initiative Group where he is investigating roles of Hsp90 chaperone machinery in DNA repair.
Clinical Research
In a clinic-laboratory collaboration with Drs. Avraham Eisbruch and Shruti Jolly, Dr. Nyati is working to understand how a specific antibody (cetuximab) that binds with EGFR produces a clinical response in certain patients with head and neck squamous cell carcinoma but fails in other patients. Using patient samples taken before and during Cetuximab treatment, they are analyzing pharmacodynamic changes in EGFR and its associated signaling molecules, epigenetic markers, HPV status and immune response molecules. They then examine the relationships between each of these factors and the overall efficacy and toxicity of cetuximab treatment. The goal of this work is to identify patients most likely to respond to cetuximab therapy in combination with chemotherapy and/or radiotherapy early in the course of treatment.
Translational Research
Dr. Nyati is working towards developing a novel agent that induces EGFR protein degradation by blocking EGFR dimer formation. Towards this, he developed a small peptide “Disruptin” that showed activity in TKI resistant lung tumor xenografts. He is currently developing small molecules that can degrade activated EGFR or mutant KRAS by blocking the protein-protein interactions that promote the stability of these oncoproteins. Click here for more information.
Dr. Nyati is also working towards the development of a strategy to treat cetuximab-resistant, KRAS-positive colorectal cancer patients.
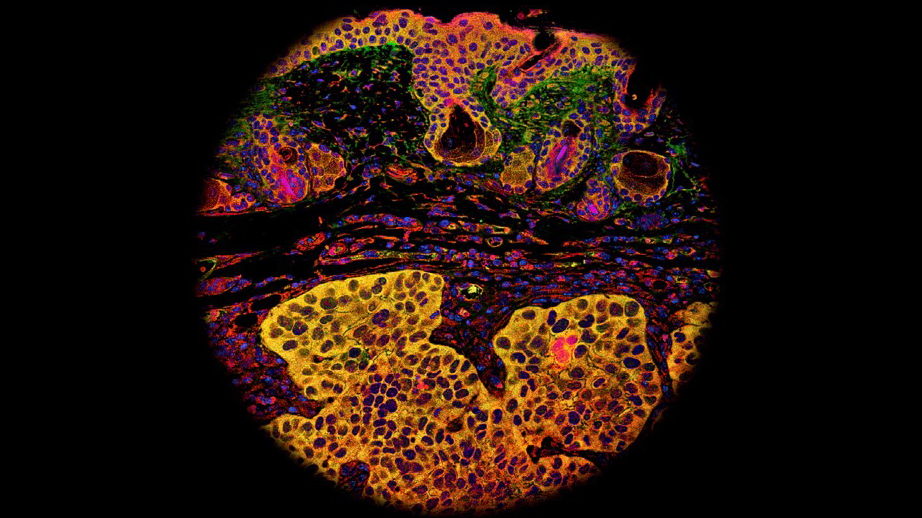
The photomicrograph shows that a cell surface receptor EGFR (red) can regulate the progression of cancer. The EGFR molecule forms a dimer upon binding with its ligand or forms a complex with Hsp90 (yellow). These interactions are required for EGFR protein stability. This image is from a tumor xenograft along with connective tissue (middle) and normal skin (top) that show higher EGFR and Hsp90 co-localization in tumors (lower half). Drs. Nyati and Lawrence found that a synthetic peptide (Disruptin) can inhibit both the dimerization of EGFR as well as its binding with Hsp90, which accelerate degradation of activated EGFR and kills EGFR driven tumor cells.
Dr. Nyati is also an avid photographer (see Medical Campus in Moonlight above) with photos published in a wide range of journals, calendars and newspapers. He has secured funding for research from a variety of sources and published articles in Nature, Science, PNAS, Cancer Research, and JBC. Dr. Nyati has trained students, post-doctoral fellows and residents in the laboratory setting. He is also a co-founder of Pi Squared Therapeutics, LLC, a start-up biotechnology company focused on selective targeting of protein stability of activated oncogenes by disrupting protein-protein interactions required for cancer cell survival.
Areas of Interest
- Signal transduction
- Protein degradation
- Radiation sensitization
- Experimental therapeutics
Honors & Awards
2014 Panelist in the first “Protein Folding Symposium”, organized at the Univ of Michigan, Ann Arbor
2013 Co-chair on receptor tyrosine kinase in Head and Neck Cancer SPORE Workshop, Rockville, MD
2010 Program committee member for the 2010 AACR Annual Meeting
2010 International examiner to review a PhD thesis entitled "Preclinical studies on the antagonistic effect of Zigerone, a phenolic alkanone against gamma radiation" for this University of Manipal, India.
2010 Panelist during AHNS Research workshop on the Biology, Prevention & Treatment of Head and Neck Cancer in Arlington, Virginia on CTEP committee member on AT13387 project team.
Credentials
- Post-doctoral Fellowship, University of Michigan, 1999-2001, Ann Arbor, MI
- Post-doctoral Fellowship, Indian Institute of Science, 1997-1998, Bangalore, India
- PhD, University of Rajasthan, 1991-1996, Jaipur, India
- MS, University of Ajmer, 1989-1991, Ajmer, India
- BS, University of Ajmer, 1986-1989, Ajmer, India
Grants
CA248310-03S1: Development of a first-in-class mEGFR dimerization inhibitor
Mukesh Nyati (PI)
04/2022-03/2025. $1,170,000
LC210305: Investigation of a Novel non-PROTAC small molecule Degrader of Activated-EGFR Against Osimertinib Resistant Non-Small Cell Lung Cancers Department of Defense
Mukesh Nyati (PI)
04/2022-04/2024. $940,564
5 R01 CA248310-05: Development of a first-in-class mEGFR dimerization inhibitor.
Mukesh Nyati (PI)
04/2020-03/2025. $2,096,110
U069216: Modulate chaperone machinery to enhance EGFR protein ubiquitination and degradation -Dr. Nyati co-developed the idea, preliminary data in a multidisciplinary team. University of Michigan Fast Forward Program
Mukesh Nyati (Co-PI)
12/2019-06/2022. $320,000 ($20,000)
5 U01 CA216449-05: Sensitization to Chemoradiation by Therapeutic Targeting of the DNA Damage Response -Dr. Nyati developed a seed grant and generated preliminary data.
Mukesh Nyati (Co-Investigator)
04/2017-03/2023. $2,990,836 ($373,148)
Published Articles or Reviews
Selected from over 50 publications
- Mehta RK, Shukla S, Ramanand SG, Somnay V, Bridges AJ, Lawrence TS, Nyati MK. Disruptin, a cell-penetrating peptide degrader of EGFR Cell-Penetrating Peptide in Cancer Therapy. Translational Oncology. 2021;14(8). doi: 10.1016/j.tranon.2021.101140.
- Mehta RK, Pal S, Kondapi K, Sitto M, Dewar C, Devasia T, Schipper MJ, Thomas DG, Basrur V, Pai MP, Morishima Y, Osawa Y, Pratt WB, Lawrence TS, Nyati MK. Low-Dose Hsp90 Inhibitor Selectively Radiosensitizes HNSCC and Pancreatic Xenografts. Clin Cancer Res. 2020;26(19):5246-57. doi: 10.1158/1078-0432.Ccr-19-3102.
- Ahsan A, Ray D, Ramanand SG, Hegde A, Whitehead C, Rehemtulla A, Morishima Y, Pratt WB, Osawa Y, Lawrence TS, Nyati MK. Destabilization of the Epidermal Growth Factor Receptor (EGFR) by a Peptide that Inhibits EGFR Binding to Hsp90 and Receptor Dimerization. J Biol Chem. 2013;288(37):26879-86. Epub 2013/07/31. doi: M113.492280 [pii]
- Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nature Reviews Cancer. 2006;6(11):876-85. doi: 10.1038/nrc1953.

