Much of our work has focused on how cells in the pancreas transform into precursor lesions and into cancer. A key player in this transformation are mutations in the KRAS gene and the K-ras protein it encodes. A mutated form of the KRAS gene is present in precursor lesions, known as Pancreatic Intraepithelial Neoplasia or PanINs. But the K-ras mutation alone isn't enough for tumor cells to grow and progress; our work has shown that they survive and thrive by reprogramming their microenvironment, which includes tumor stroma, or connective tissue, and the extracellular matrix. Using a range of analytical platforms and tools, we continue to elucidate how this reprogramming occurs through the signaling and crosstalk among tumor cells and their microenvironment. Looking at these processes reciprocally is a hallmark of our current work, and the murine models and analytic platforms we have developed and optimized let us perform characterizations of cell, tissue and organoid models at much higher resolution than ever before.
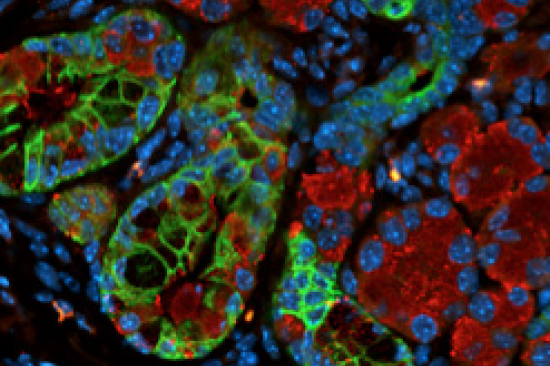


Overview
Strategies
Our laboratory uses a number of strategies to investigate the biology and immunology of pancreatic cancer:
- Clarifying the role of mutated Kras proteins in reprogramming the tumor microenvironment and identifying the many cell signaling pathways involved. Since KRAS plays an important role in cancer initiation, maintenance and progression, and since no agent currently exists to inhibit it, we also are identifying potential alternative downstream targets.
- Understanding macrophage biology and interactions in pancreatic cancer as well as looking at the role of T cells in immune suppression. The pancreatic cancer microenvironment typically shows a significant infiltration of immune cells, including T cells, but they have a suppressive rather than protective effect.
- Participation in a national collaboration focused on interrupting the cellular crosstalk that leads to immunosuppression in the pancreatic cancer microenvironment.
Translational work to characterize interactions within tumors using three-dimensional or organoid cultures and single-cell sequencing technology.
Results
Among many other discoveries, our investigators were the first to explain the role of the Hedgehog signaling pathway as well as its interaction with Ras signaling in the development of pancreas cancer. Our early work also elucidated the roles of Wnt and Notch signaling in cancer initiation and progression. Mutations in the KRAS gene have been associated with the development of pancreas cancer, and our group was the first to show that KRAS also is required for ongoing tumor survival and metastasis. Our work further found that impaired Kras proteins impact multiple cells types in the tumor microenvironment, including infiltrating myeloid cells, creating more suitable surroundings to support tumor maintenance. Work to understand epigenetic changes in the context of pancreatic cancer include our discovery of the necessary role of the Bmi1 protein in pancreas cancer initiation. Research on the ATDC gene has shed new light on pancreatic cancer initiation and provides evidence of a potential new target for thwarting early processes that lead to tumor development.
Clinical Relevance & Impact
Our work is advancing the understanding of the many and complex mechanisms underlying pancreatic cancer development, maintenance and progression. Our findings are moving us from the realm of biology to novel, translational precision-medicine approaches that can guide treatment planning, decision-making and evaluation.
Future Directions
The Pasca di Magliano Laboratory is excited to be initiating several translational projects using our optimized analytical platforms. These are allowing us to extract much more information than ever before from our murine models, human tissue samples and three-dimensional organoid models. Our laboratory is also heavily involved in training and mentoring individuals at all levels, from graduate students to postdoctoral researchers, residents and junior faculty. Educating future investigators is the core mission of all that we do.
Collaborations
Collaboration is crucial to our work, and the University of Michigan offers a uniquely collaborative environment for studying — and translating — pancreatic cancer research.
- In addition to our laboratory, other investigators from the Department of Surgery participate in the Pancreatic Cancer Research Initiative (PCRI). Collaborators in the PCRI hail from across the University and beyond, from specialists in internal medicine, cellular and molecular biology and pharmacology to hematology, oncology and computational medicine and biology.
- Our laboratory, as well as other Department of Surgery faculty, also participate in a six-center consortium funded by the National Cancer Institute. The Pancreatic Cancer Microenvironment Network (PACMEN) aims to better understand the role of the tumor microenvironment and how it leads to resistance to immunotherapy treatment.
- With Lonnie Shea, the William and Valerie Hall Chair of Biomedical Engineering, our team is looking at the signaling mechanisms tumor cells use to recruit immune cells. This new approach utilizes engineered scaffolds and is helping us learn more about the process of metastasis in pancreatic cancer.
- We are part of many other ongoing collaborations, including with the Center for Organogenesis and other faculty laboratories, to help spur discovery and generate new approaches to treating and preventing this disease.
Funding
- National Cancer Institute, U01: PAncreatic Cancer MicroENvironment Network (PACMEN)
- American Cancer Society Research Scholar Grant


