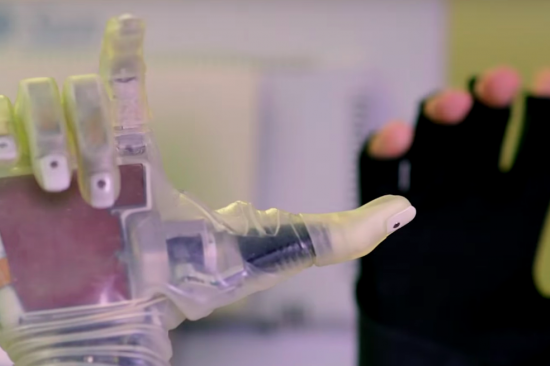
Until we understand how to provide prosthetic limbs with intuitive afferent somatosensory feedback — essential for interacting with one’s environment — while simultaneously providing efferent signals for prosthetic control, the ideal prosthetic simply cannot exist. The shortfalls eventually lead to a high degree of abandonment of prosthetic devices, no matter how technologically advanced they may seem.
Peripheral nerve interfaces for controlling upper-extremity prosthetic devices are not novel, but approaches to date have required electrodes to be placed around or within the nerve itself. However, these interfaces have proven unstable. They often damage the peripheral nerve, and the signal degrades over time. Moreover, they are not effective at preventing neuroma formation or recurrence.
Instead, our approach involves creating a regenerative peripheral nerve interface (RPNI) construct by implanting a residual peripheral nerve into an autologous muscle graft that has been denervated and devascularized. An electrode records signals from the nerve but can be implanted in the muscle, thereby preventing nerve damage. The grafts serve as biologic targets within which the nerve ends can regenerate and signal. In fact, we have observed rapid and robust formation of new neuromuscular connections within the interface in our experiments. As we performed this research, first in small and large animal models and then in humans, we saw that no new neuromas formed in these cases, and we began to investigate the use of RPNIs in both neuroma treatment and prevention.