In the sub-basement of a building on the U-M’s medical campus, 20 people in blue scrubs and white coats crowd into a surgical suite in the U-M’s Extracorporeal Life Support Research Laboratory. They are learning how to evaluate lungs for potential transplantation outside the body during a process called ex vivo lung perfusion.
These surgeons and technicians are preparing for a groundbreaking clinical trial at the U-M Health System. Today they are training with animal lungs, but once the study begins, they will be working with human lungs. Called the Novel Lung Trial, it could mean the difference between life and death to thousands of people who need a lung transplant.
The trial is sponsored by XVIVO Perfusion, the Swedish-based manufacturer of a machine called an XVIVO Perfusion System (XPS) and the perfusion solution called STEEN Solution, both used in the study. After surgeons remove lungs and attach them to the machine, the XPS flushes them with STEEN Solution and warms them to normal body temperature, inflates the lungs to their normal size and then tests them to determine if the lungs are healthy enough to transplant. The environment inside the system can allow some damaged lungs to heal themselves, or recondition. The machine’s function, though, is to allow physicians to evaluate a lung for transplant.

Ex vivo lung perfusion is a direct descendant of a life-saving procedure called extracorporeal membrane oxygenation (ECMO), which was developed by U-M surgeon Robert Bartlett (M.D. 1963) and his colleagues during the 1960s and 1970s to treat children dying from acute respiratory failure. The device, invented by a small group of young surgeons to heal sick lungs inside the body, has evolved into a machine that can give damaged lungs a chance to heal outside the body.
It’s the latest in a long line of research advances taking place in the U-M’s Extracorporeal Life Support (ECLS) Research Laboratory. Bartlett, now a professor emeritus of surgery, brought the lab to U-M in 1980 when he joined the Medical School’s faculty. Part of the Department of Surgery, the lab has an international reputation for pushing the boundaries of knowledge about artificial organs and organ transplants.
“This laboratory has always been focused on the big clinical problems and what we can do to solve them quickly,” says Bartlett, who has an enviable record of 40 consecutive years of NIH research funding. “We can’t always do it within five years, but that is always our goal.”
Advances in ECLS technology are bringing us closer to what Bartlett says is the field’s ultimate goal. It’s called organ banking or organ conditioning. The objective is to keep human organs viable and healthy outside the body for several days — much longer than the current limit of a few hours. Once researchers figure out how to do this, Bartlett says ECLS will revolutionize the practice of medicine.

“Not only could we have many more organs available for transplantation, we could have organs that do things — livers that make albumin and clotting factors, bone marrow that makes red blood cells,” says Bartlett. “We could take out a liver that’s full of cancer, treat it and then return it to the patient. We think all those things could be possible.”
Advanced ECLS technology is already being used clinically in Canada and Europe either as a temporary bridge to lung transplant, or to evaluate health of lungs awaiting transplant. Researchers are developing technology to support other organs like hearts, livers and kidneys outside the body, but this work is still in the animal research stage.
Bartlett is not the only U-M physician who is enthusiastic about what ECLS research could mean to thousands of patients on the waiting list for a donor organ and to the doctors who care for them.
“I think that the number of lungs available for transplant will be 50 percent greater five to 10 years from now, because of this technology,” says Jeffrey Punch (M.D. 1986, Residency 1992, Fellowship 1994), a U-M professor of surgery and head of the Section of Transplantation at UMHS. “I hope we’ll see renewed interest in doing this for livers and kidneys, as well.”
Bartlett has been caring for sick and damaged lungs since the 1960s, when he and his fellow surgeons at Boston Children’s Hospital invented a modified heart-lung machine and a procedure they called ECMO. After years of testing the procedure on research animals, Bartlett began using ECMO in the 1970s to treat infants and children who were dying from acute respiratory failure.
ECMO’s successful use in human patients would never have been possible without animal research. Large animals, especially sheep and pigs, were vital to Bartlett’s research, because the organs are similar in size and structure to those of a human being. Animal research remains the cornerstone of advances in ECLS technology today. Investigators at the U-M, like those at other federally-funded research institutions, must follow strict regulations for the humane and ethical treatment of all animals used in research.
The first ECMO machines were big, cumbersome devices cobbled together from component parts. The machine worked by taking over temporarily for the patient’s lungs to give them time to rest and recover. A large plastic tube inserted in the patient’s jugular vein diverted blood away from the lungs and into the ECMO machine where it passed through a membrane filter that removed carbon dioxide and added oxygen. Oxygen-rich blood was then pumped from the machine back into the patient’s circulatory system via the carotid artery.
After he moved to the U-M, Bartlett began using ECMO to treat adults, as well as children. After clinical trials proved that ECMO was safe and effective, it became the standard of care for adults with acute heart, lung and kidney failure. As more physicians began using ECMO, equipment manufacturers started developing and marketing more sophisticated machines.
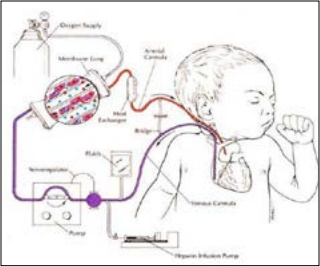
Today, Bartlett says more than 60,000 patients at 350 medical centers around the world have been treated with ECMO. Among them are three U-M students who have recently worked in the ECLS lab and were saved as babies. They have helped advance the technology that saved their lives years ago. Just as the lab’s medical legacy is significant, so too is its impact on medical training. Each year, about 100 people — from undergraduates to post-docs — work in the ECLS lab, pushing the technology further.
ECMO is very good at treating acute lung disorders. But it can’t help patients with chronic progressive lung diseases like COPD, pulmonary fibrosis or cystic fibrosis. Eventually these patients are left with just one option: a lung transplant.
As of May 16, there were 1,648 Americans with severe lung disease on the waiting list for a lung transplant – according to the Organ Procurement and Transplantation Network, the organization that maintains the national transplant registry.
These people have more than a casual interest in extracorporeal life support research. For them, it’s a matter of life and death. They need a transplant to survive, but there aren’t nearly enough donor organs to go around. The shortage is especially acute for lungs. Yet about 80 percent of lungs from potential donors are thrown away, because surgeons don’t want to risk transplanting even slightly damaged lungs into an already fragile patient.
“Compared to a kidney, the lung doesn’t respond to injury well, and the consequences of a lung not working after a transplant are much greater,” explains Punch. “This is why the criteria for donor lungs are so stringent and why most lung donors are young people who had relatively short illnesses before they died.”
Lungs are fragile and can be damaged in many ways. Fluid can accumulate in the lungs leading to pneumonia. Traumatic injuries and emergency CPR procedures can leave bruises on delicate lung tissue. And lungs are particularly susceptible to immune system rejection after a transplant.

Transplant surgeons agree that many of these so-called marginal lungs could work. The problem is there’s no way to determine in advance which ones are safe to transplant and which ones aren’t.
This is why the Novel Lung Trial is so important. Results from this nationwide clinical trial will help the U.S. Food and Drug Administration determine whether marginal human lungs can be evaluated within the XVIVO Perfusion System and made safe for transplant.
“With this device, we can take lungs for a test drive before transplanting them into a patient,” says William R. Lynch (M.D. 1994, Residency 2003), an associate professor of surgery who was recruited to the Medical School to lead the clinical trial and direct a new U-M research program on ex vivo lung perfusion. Lynch trained with Shaf Keshavjee, M.D., the University of Toronto surgeon who pioneered the development of ex vivo lung perfusion and was the first in North America to perform a clinical ex vivo lung transplant.
The $250,000 XPS system to be used in the study was purchased by Gift of Life Michigan – the state’s organ procurement organization. Access to the machine will make it possible for Michigan’s three organ transplant centers – U-M Health System in Ann Arbor, Henry Ford Health System in Detroit and Spectrum Health in Grand Rapids – to join the XVIVO trial, which is currently underway at six academic medical centers across the United States.
When the trial is complete, the FDA will compare outcomes of patients who received a conventional lung transplant to outcomes of patients who received lungs on which doctors used the XVIVO system. The study’s results will determine whether less-than-perfect human donor lungs are approved for transplant in the United States, as they are currently in Canada and Europe. Lynch is enthusiastic about the trial’s potential to increase the pool of donor lungs, so more people who need a lung can get one.
“We do about 1,800 lung transplants in the United States per year,” says Lynch. “There are a lot more than 1,800 people dying in our emergency rooms. If we could recondition just half the lungs from deceased people in our ERs, we could triple the number of lung transplants.”
As principal investigator for the upcoming clinical trial at UMHS, Lynch is responsible for making it all work. It’s his job to ensure that surgeons and technical staff from three different institutions maintain their skills and learn to work together as a team, so they’ll be ready when it’s time for Michigan’s first transplant of an ex vivo lung.
The scientific and technical hurdles are daunting enough, but Lynch also worries about cultural and organizational barriers that could make it more difficult for these organs to be accepted here than they are in other countries. In the United States, for example, surgeons can’t recover organs from someone who has died without their advance consent and/or the consent of the family. In Spain, on the other hand, the body of a dead person belongs to the state and consent for organ donation is assumed, unless someone opts out in advance.
Lynch says the transplant community is just beginning to consider important questions, such as: How many institutions will take the risk of transplanting donor organs that have been evaluated out of the body? How should this expanded pool of organs be shared? How will we pay for this new ex vivo technology? It costs $10,000 to $20,000 per perfusion and, as Lynch points out, the lungs don’t have medical insurance.
Resolving these complex cultural and economic issues will not be easy, but it’s time to get started, because one fact is clear: After decades of hard work and millions of research dollars invested in ECLS technology, we are closer than ever to the day when no one needs to die for lack of a donor organ. Making that day a reality, with approaches like ex vivo lung perfusion, would be a fitting legacy to the journey Bartlett and his colleagues began in the 1960s. [M]
The Future of Life Support
Thirty-four years of research in the U-M’s ECLS lab have generated many new applications for ECMO. Industrial partners have collaborated with researchers to develop next-generation ECMO machines that are simpler, safer and easier to use.
Today, researchers are using this advanced ECMO-based technology to support lungs, hearts, livers, kidneys and other organs outside the body for extended periods of time.
Several innovative research projects are currently underway in the ECLS lab:
• Only about 5 percent of people who go into cardiac arrest in an emergency room or hospital survive without major brain damage. Studies have shown that adding ECMO to CPR can increase survival rates to 40 percent. Emergency medicine physicians at U-M are developing an ECMO/ CPR protocol and training materials for physicians and nurses to use in an emergency. The goal is to get cardiac arrest patients on ECMO immediately so more patients can survive and recover.
• UMHS has extensive experience in using ECMO to maintain the abdominal organs of brain-dead patients after life support is withdrawn. This use of ECMO allows physicians to preserve the patient’s kidneys, liver, pancreas and lungs for several hours after all heart and brain activity has stopped. This gives the family and transplant team more time to make arrangements for organ donation and may improve the outcome of organs that are donated under these circumstances.
• Bartlett and Mark Meyerhoff, Ph.D., the Philip J. Elving Collegiate Professor of Chemistry, have discovered a molecule that prevents blood platelets from sticking to the surface of plastic devices. This discovery could solve one of ECMO’s biggest problems – the fact that blood will clot when it touches an artificial surface, like the plastic tubing used to connect patients to an ECMO machine. Patients on ECMO take anticoagulant drugs to prevent clot formation, but these drugs increase the risk of uncontrolled bleeding. Bartlett hopes that polymers seeded with the new molecule will be available for clinical applications within two or three years.
• The artificial placenta is a new application of ECMO-based technology that’s still in the animal research stage. The device could one day help premature infants who are born before their lungs develop. Today, these tiny premies must be intubated to force oxygen into their immature lungs, which can cause severe side effects. The artificial placenta reproduces normal fetal blood circulation by bypassing undeveloped lungs completely – pumping oxygenated blood directly to the heart.
• Finding the right donor heart for transplant is hard enough with an adult patient, but finding the right pediatricsized heart in time to save a sick child’s life is even more difficult. U-M pediatric cardiologists hope to use ex vivo technology to extend the time hearts can be maintained outside the body giving them more time to locate the best match for a heart transplant.
This story, written by Sally Pobojewski, originally appeared in Medicine at Michigan magazine. Illustration by Ann Cutting.
Original Article: http://www.medicineatmichigan.org/sites/default/files/archives/bringing-lungs-bloom.pdf

Robert H. Bartlett, MD

William R. Lynch, MD


