Available to mentor

I grew up in Northern Kentucky and attended Northern Kentucky University as an undergraduate student. During this time, I worked at the Wood Hudson Cancer Research Laboratory with Dr. Bonnie Richmond and later in the lab of Dr. Patrick Schultheis at Northern Kentucky University. I graduated with two BS degrees in 2007 (in Biological Sciences and Chemistry). My PhD training was with Todd Graham at Vanderbilt University where I worked on mechanics of phospholipid "flippases", graduating in 2013. I completed my postdoctoral training with Tom Rapoport at Harvard Medical School in 2017, working on mechanics of the the endoplasmic reticulum associated degradation system (ERAD). I started my lab at the University of Michigan Medical School in September 2017.
Baldridge Lab
-
Damon Runyon Cancer Research Foundation FellowHarvard Medical School, Cell Biology, 2017
-
PhDVanderbilt University, Nashville, 2013
-
BSNorthern Kentucky University, Highland Heights, 2007
-
BSNorthern Kentucky University, Highland Heights, 2007
We are interested in the basic mechanisms of protein quality control systems at cellular membranes. We study how these systems select their substrates, and aim to identify the cellular pathways regulated by them. These processes are important in pathologies related to cell stress, protein misfolding, and protein misregulation. Human illnesses linked to these problems include Parkinson’s disease, Alzheimer’s disease, and various cancers. Our longer-term goals are to understand how changing conditions in cells target substrate proteins to these integral-membrane quality control systems and exploit these systems to enable targeted protein degradation from cellular organelles.
-
Peterson BG, Hwang J, Russ JE, Schroeder JW, Freddolino PL, Baldridge RD. Cell Rep, 2023 Nov 28; 42 (11): 113451Journal ArticleDeep mutational scanning highlights a role for cytosolic regions in Hrd1 function.
DOI:10.1016/j.celrep.2023.113451 PMID: 37980570 -
Sharninghausen R, Hwang J, Dennison D, Baldridge RD. 2023 Sep 5; eLife Sciences Publications, Ltd,PreprintIdentification of ERAD-dependent degrons for the endoplasmic reticulum lumen
DOI:10.7554/elife.89606 -
Assainar BM, Ragunathan K, Baldridge R. 2023 bioRxiv,PreprintDirect observation of autoubiquitination for an integral membrane ubiquitin ligase in ERAD
DOI:10.1101/2023.06.20.545802 -
Hwang J, Peterson BG, Knupp J, Baldridge RD. Sci Adv, 2023 Jan 13; 9 (2): eadd8579Journal ArticleThe ERAD system is restricted by elevated ceramides.
DOI:10.1126/sciadv.add8579 PMID: 36638172 -
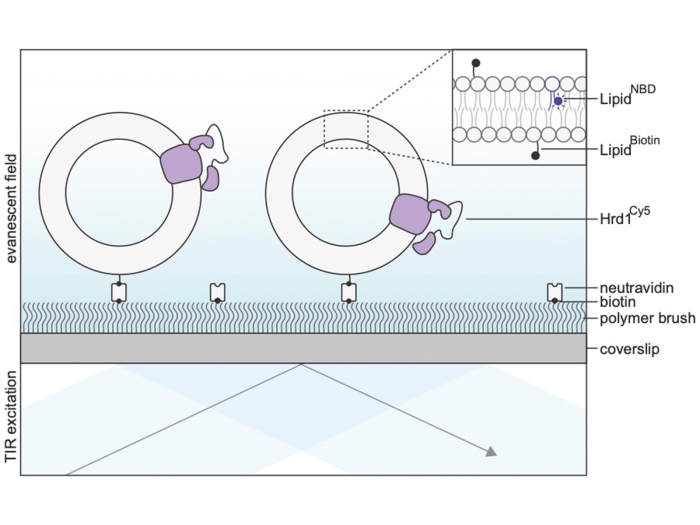
Peterson BG, Glaser ML, Rapoport TA, Baldridge RD. Elife, 2019 Nov 12; 8:Journal ArticleCycles of autoubiquitination and deubiquitination regulate the ERAD ubiquitin ligase Hrd1.
DOI:10.7554/eLife.50903 PMID: 31713515 -
Baldridge RD, Rapoport TA. Cell, 2016 Jul 14; 166 (2): 394 - 407.Journal ArticleAutoubiquitination of the Hrd1 Ligase Triggers Protein Retrotranslocation in ERAD.
DOI:10.1016/j.cell.2016.05.048 PMID: 27321670 -
Baldridge RD, Xu P, Graham TR. J Biol Chem, 2013 Jul 5; 288 (27): 19516 - 19527.Journal ArticleType IV P-type ATPases distinguish mono- versus diacyl phosphatidylserine using a cytofacial exit gate in the membrane domain.
DOI:10.1074/jbc.M113.476911 PMID: 23709217 -
Baldridge RD, Graham TR. Proc Natl Acad Sci U S A, 2013 Jan 29; 110 (5): E358 - E367.Journal ArticleTwo-gate mechanism for phospholipid selection and transport by type IV P-type ATPases.
DOI:10.1073/pnas.1216948110 PMID: 23302692