A new initiative, funded by Michigan Medicine’s own Department of Neurology, brings together the past, present, and future of Eva Feldman M.D., Ph.D.’s legacy. A former mentee of Dr. Feldman, and now diabetes wunderkind, Rodica Pop-Busui, M.D., Ph.D., joins current NeuroNetwork faculty Stephanie Eid, Ph.D., along with Martin Meyers, M.D., Ph.D., to investigate the intersection of genetics and diabetic neuropathy through a grant titled “Role of the voltage-gated sodium channel gene Scn2a in diabetic peripheral neuropathy.” The funding’s purpose is to support innovative ideas that don’t have a ton of preliminary data. This support from the Department of Neurology looks to allow researchers to gather this preliminary data and go after much larger grants.

The Neuropathy Problem
In the United States, 1 in every 10 adults has diabetes, and over 20% of those don’t even know they have it. Type 2 diabetes, the more common form, accounts for around 95% of diabetes cases and is diagnosed most frequently in adults. It is well established that diabetes brings with it a host of complications hitting various areas of the body, including diabetic peripheral neuropathy (DPN), a debilitating condition for which there is no effective treatment. Genetic factors have been implicated in DPN, but their identities and mechanisms are mostly unknown.
A collaboration is formed.

Dr. Pop-Busui was involved in a large genome-wide association study (GWAS) that analyzed two large, well-characterized cohorts of type two diabetes patients. In a GWAS, the idea is to determine genes that are associated with disease pathogenesis or progression, in this case, DPN. Through this work, Dr. Pop-Busui found that the gene SCN2A was associated with DPN progression. Specifically, they found that it was present in patients that did not have DPN and absent in patients with DPN. Because of this fact, they predicted that this gene is protective of nerves.
In the past, the SCN2A gene has been studied with epilepsy, Parkinson’s disease, autism, and other neurodevelopmental disorders associated with the central nervous system, but nothing is known about its role in DPN or the peripheral nerves. Dr. Pop-Busui was the first to make this connection.
Dr. Pop-Busui sought us (NeuroNetwork for Emerging Therapies) out due to our expertise in DPN research, and Dr. Meyers, who specializes in genetically modified pre-clinical models, to further study how SCN2A protects neurons in diabetes.

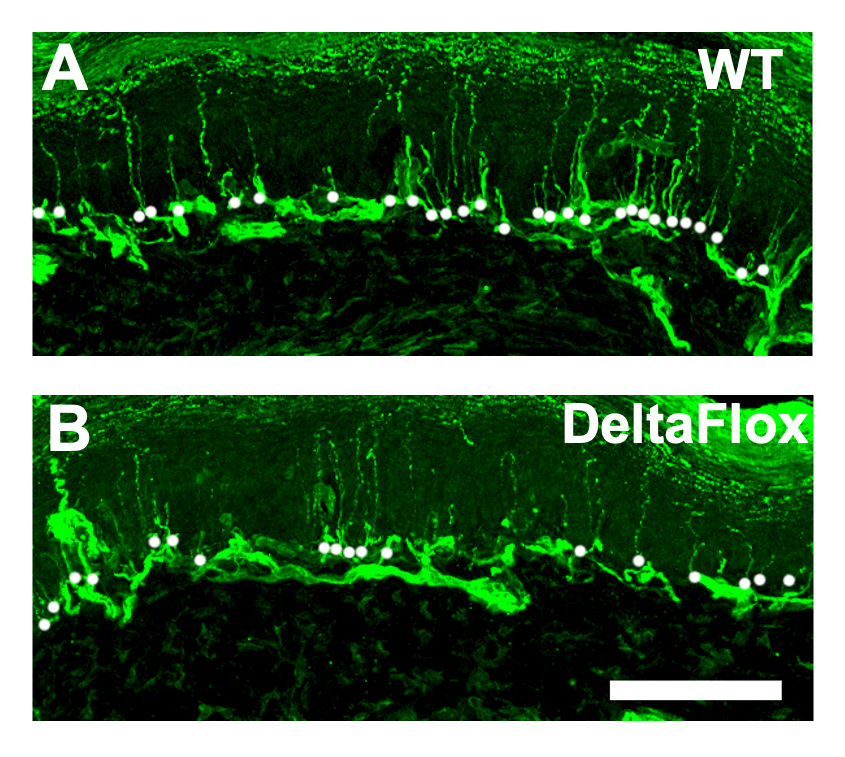
The pre-clinical model that Dr. Meyers’s team generated was created not to express SCN2A, the gene of interest, particularly in sensory neurons. They were especially interested in sensory neurons because they are a target of neuropathy. Furthermore, SCN2A is known to be exclusively expressed in sensory neurons, not in any other peripheral nervous system cell type.
Initial results are promising.
Our initial analysis found that when we delete SCN2A from sensory neurons in pre-clinical models without neuropathy (controls that lack the gene), that by itself reduces distal nerves or cutaneous fibers. This means that this gene by itself is important for the growth of nerves under normal conditions. Basically, it confirmed Dr. Pop-Busui’s conclusions from her clinical studies that SCN2A is important for normal peripheral nervous system functioning.
Now, we need to investigate the diabetes context.
We will take the pre-clinical model from Dr. Myers with the SCN2A gene deleted and induce type 2 diabetes. This will be done by feeding it a high-fat diet and causing hyperglycemia, which together will create the type 2 diabetes model.
- Does diabetes alone affect the level of SCN2A?
- If we delete the gene and add diabetes, does it worsen neuropathy?
How does this help diabetes patients?
The hope of this study is to provide insight into the genetic basis of diabetic neuropathy and allow us to look further into the mechanisms implicated. In other words, how is SCN2A leading to injury?
A better understanding of these areas will help us identify higher neuropathy risks and new therapeutic targets. Right now, there is no effective treatment for neuropathy, which can be a very debilitating disease.
Primary investigator on the grant and Rose C. and Nathan L. Milstein Family Emerging Scholar Dr. Eid explained one of the possibilities:
“Definitely further down the line, we will have to manipulate SCN2A, to see if we can worsen the condition or further protect the peripheral nerves. If it turns out that this gene is really necessary for normal nerve function, there is the possibility of creating a therapy that could overexpress SCN2A, to see if you can improve nerve function. Right now, there isn’t anything out there that would do this for neuropathy patients. This could be the start of a new hope for patients.”
