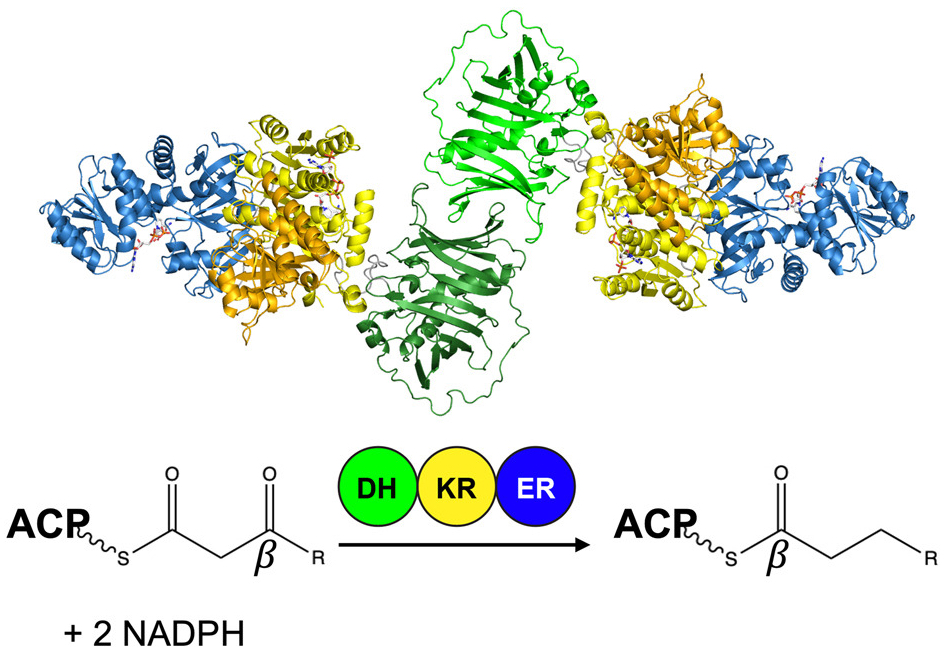
Polyketides are a chemically diverse class of natural products that form the scaffolds of numerous therapeutics, and many are assembled by modular polyketide synthases (PKS) from bacteria. In work soon to be published in Structure, researchers in the laboratory of Janet Smith present the structure of the entire reducing region from a modular PKS: the ketoreductase (KR), dehydratase (DH), and enoylreductase (ER) domains of module 5 of the juvenimicin PKS. The structure illustrates the divergence of modular PKS from iterative megasynthase homologs including metazoan fatty acid synthase and reveals structural elements shared by PKS modules with fully reducing tridomains. Tyler McCullough is the lead author of the new paper and is also the department's most recent graduate (Ph.D. '23).
Read the press release from UM Life Sciences Institute HERE.