Age-Related Hearing Loss: Characterization & Treatments
Age-related hearing loss is the most prevalent form of hearing loss in humans, affecting close to a third of people over the age of 65. A decreased ability to communicate reduces quality of life, increases the risks from other age-related changes and illnesses and contributes to the incidence of depression and dementia. Our previous studies have characterized age-related cellular and synaptic changes in the mouse cochlea, resulting physiological and behavioral effects and identified potential underlying molecular mechanisms and genetic factors that contribute to these changes (Altschuler et al., 2016; Schacht et al., 2012; Sha et al., 2008). Most recently we have identified treatments shown to increase mouse lifespan in National Institute on Aging Intervention Testing Program studies that we find will also reduce / delay age-related loss of sensory cells (outer hair cells) in the mouse inner ear. Current studies are testing the influence of adding rapamycin (acting on mTOR pathways) or ACEMg (mixture of anti-oxidants) to diet at 10 months of age on various components of later occurring age-related hearing loss. We will also investigate the specific molecular mechanisms underlying effects.
Noise Induced Vestibular Disorder
There is increasing evidence that noise can cause vestibular dysfunction and balance disorder. Our recent studies in the rat model show that noise of varying intensity and duration can cause changes in vestibular nerve physiology as measured by vestibular short-latency evoked potentials (VsEP) and changes in vestibular hair cell – vestibular nerve synaptic connections (Stewart et al, 2018) that result in changes in vestibular associated behavior such as on balance beam. These changes can be short-term or persist. Our current studies are investigating the relationship between physiological/functional measure and cellular and changes in calyceal versus non-calyceal synaptic connections between hair cells and vestibular nerve. We are also investigating oxidative stress and treatments that could prevent or reverse synaptic changes. We are coordinating studies with collaborators doing human studies and examining noise-associated vestibular changes and disorders in veterans.
Noise-induced Tinnitus: Characterization, Prevention and Treatments
Tinnitus is the “phantom” perception of a sound, in the absence of that sound, often perceived as a ringing in the ear(s). Tinnitus can generate depression, anxiety, insomnia and reduce quality of life. It occurs in over 15% of US adults with many needing professional assistance to cope. Approximately 40% of all veterans report tinnitus and it is the number one co-reported service-related disability for those who served in the Gulf Wars. We have used reduced Gap Detection and operant conditioning responses as metrics for noise-induced tinnitus in the rat model, testing a small arms-fire like noise as well as broad band noise overstimulation. We find these noises can induce tinnitus is a sub-set (30-50%) of noise exposed animals. We find increased loss of synaptic connections between inner hair cells and the auditory nerve in the rats testing positive for tinnitus according to our metrics and are testing the hypothesis that preventing this loss or inducing re-connection of lost synapses will reduce the incidence of tinnitus. Current studies are testing this hypothesis using anti-excitotoxicity treatments to prevent loss or administration of neurotrophic factors to induce reconnection after loss.
Noise Induced Hearing Loss: Characterization & Treatments
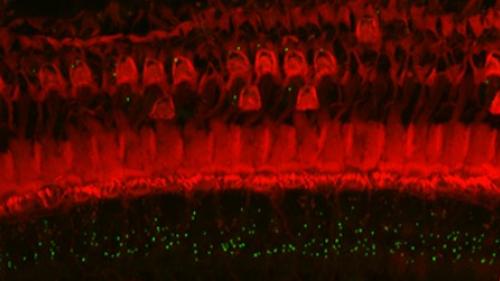
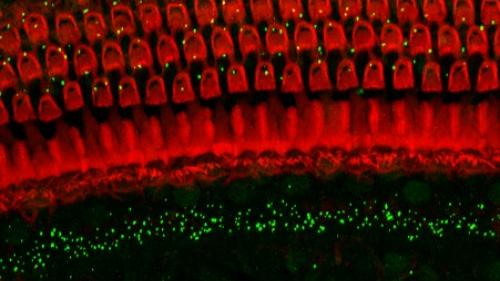
Noise is one of the most common causes of hearing loss. Depending on the characteristics of the noise (e.g, intensity and duration) and individual susceptibility the effects can be temporary or permanent. Noise can result in temporary or permanent changes in hearing thresholds, suprathreshold and dynamic range and in auditory processing such as gap detection. We are examining underlying mechanisms such as oxidative stress, ER and mitochondrial stress and excitotoxicity as well as mechanism based treatments such as anti-oxidants and anti-excitotoxicity agents. We have previously shown that several neurotrophic factors such as BDNF and GDNF can reduce noise-induced loss of sensory (hair cells) and loss of auditory nerves as well as induce regrowth of auditory nerve (e.g. Shoji et al., 2000; Miller et al., 2007; Glueckert et al., 2008). We have recently shown that anti-excitotoxicity treatments can reduce noise-induced loss of inner hair cell – auditory nerve synapses and addition of anti-oxidants makes this even more effective (Altschuler et al., 2016). We are currently studying use of neurotrophic factors to induce reconnection and repair of inner hair cell – auditory nerve connections lost following different types of noise overstimulation.
Endogenous Protective Pathways
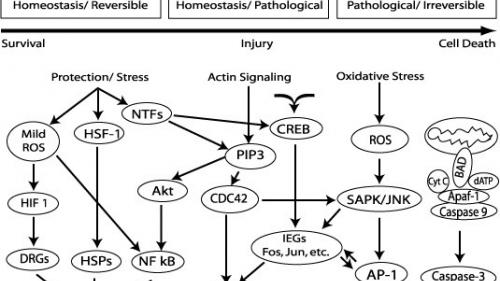
We have previously identified components of several heat shock / stress shock pathways in the cochlea and studied how they can provide protection and repair from a variety of stresses such as noise, hypoxia and ototoxins (e.g. Lim et al., 1993; LePrell et al., 2003; Fairfield et al., 2005). We have also examined anti-oxidant mechanisms in the cochlea and vestibular sensorineural epithelium (e.g. Garetz et al., 1994; Maruyama et al., 2007). We are currently studying how these pathways can be manipulated to increase the capacity of endogenous pathways/mechanisms for protection and repair.
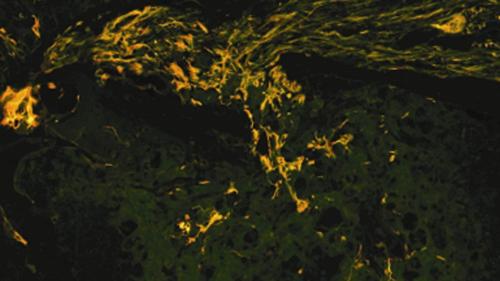
Sensorineural hearing loss will most often involve loss of cochlear hair cells and/or auditory nerve, none of which are naturally capable of regeneration in mammals. Our studies take two approaches to generating auditory neurons and hair cells. For new auditory nerve our approach is to use exogenous stem cells transplanted into the cochlea, inducing differentiation into the appropriate neuronal niche following placement into the cochlea (e.g. Altschuler et al., 2007; Reyes et al., 2008; Tong et al., 2010; Hill et al., 2012). For new hair cells our approach is to identify factors that can induce de-differentiation of cochlear supporting cells into a stem cell-like state conducive to trans-differentiation or novel division into new hair cells or to remove factors that inhibit such de-differentiation following hair cell loss (e.g. Watanabe et al., 2012)
References
Altschuler RA, Wys N, Prieskorn D, Martin C, DeRemer S, Bledsoe S, Miller JM, Treatment with Piribedil and Memantine Reduces Noise-Induced Loss of Inner Hair Cell Synaptic Ribbons, Sci. Rep. 6, 30821; doi: 10.1038/srep30821 (2016).
Altschuler RA, Dolan DF, Halsey K, Kanicki A, Deng N, Martin C, Eberle J, Kohrman D, Miller, RA, Schacht J (2015) Age-related Changes in Auditory Nerve – Inner Hair Cell Connections, Hair Cell Numbers, Auditory Brain Stem Response and Gap Detection in UM-HET4 Mice, Neuroscience 292:22-33 doi. 10.1016/j.neuroscience.2015.01.068
Altschuler, RA, O’Shea KS, Miller JM, Stem cell transplantation for auditory nerve replacement, Hearing Research, 242:110-6, 2008.
Fairfield, DA, Margaret I. Lomax, MI, Gary A. Dootz, GA, Chen, S, Galecki, TA, Benjamin, IJ, David F. Dolan, DF, Altschuler, RA, Heat shock factor 1 (Hsf1) deficient mice exhibit decreased recovery of hearing following noise overstimulation, J. Neurosci Res. 81:589-596, 2005.
Garetz S, Altschuler RA & Schacht J: Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo, Hearing Research 77:81-97, 1994
Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentiation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 507(4):1602-1621, 2008
Hill GW 3rd, Purcell EK, Liu L, Velkey JM, Altschuler RA, Duncan RK, Netrin-1 mediated axon guidance in mouse embryonic stem cells overexpressing neurogenin-1, Stem Cell and Development 21(15):2827-37 2012.
LePrell C, Dolan D, Schacht J, Miller JM, Lomax M and Altschuler RA, Pathways for Protection from Noise Induced Hearing Loss, Noise and Health, 5:20, 1-17, 2003.
Lim HH, Jenkins OH, Myers MW, Miller JM and Altschuler RA: Detection of HSP 72 synthesis after acoustic overstimulation in rat cochlea. Hearing Res. 69:146-150, 1993.
MaruyamaJ, YamagataT, UlfendahlM, BredbergG, AltschulerRA, Miller JM, Effects of antioxidants on auditory nerve function and survival in deafened guinea pigs, Neurobiology of Disease, 25(2):309-18, 2007.
Miller JM, LePrell CG, Prieskorn DM, Wys NL, Altschuler RA, Delayed Neurotrophin Treatment Following Deafness Rescues Spiral Ganglion Cells from Death and Promotes Regrowth of Auditory Nerve Peripheral Processes: Effects of Brain-Derived Neurotrophic Factor and Fibroblast Growth Factor, J NeuroSci Res 85(9): 1959-69, 2007
Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse, Hearing Res, 243:87-94, 2008
Schacht J, Altschuler RA, Burke DT, Chen S, Dolan D, Galecki AT, Kohrman D, Miller RA, Alleles that modulate late life hearing in genetically heterogeneous mice, Neurobiol Aging. 1842. 15-29 2012
Shoji, F, Miller, A.M., Yamasoba, T, Altschuler, RA, Miller, JM: Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss, Hearing Research, 146:134-142, 2000Stewart CS, Kanicki A, Altschuler RA, King WM, Vestibular short latency evoked potential (VsEP) is abolished by low frequency noise exposure in rats. J. Neurophys. 119(2):662-667. 2018
Tong M, Hernandez J, Purcell EK, Altschuler, RA, Duncan KD, The intrinsic electrophysiological properties of neurons derived from mouse embryonic stem cells overexpressing Neurogenin-1 Am J Physiol Cell Physiol.,299:C1334-44, 2010
Watanabe R, Morell MH, Miller JM, Kanicki A, O’Shea KS, Altschuler RA, Raphael Y, Nestin expressing cells in the developing, mature and noise-exposed cochlea, Mol & Cell Neuroscience 49(2):104-9, 2012