
Biography
Dr. Altschuler received his Ph.D. in Anatomy at the University of Minnesota studying effects of learning and motor experience on synapses in the hippocampus. He next moved into study of the auditory system and spent from 1978-1985 at the Lab of Neuro-Otolaryngology in the NIH Intramural Program. His studies identified and characterized neurotransmitters and receptors in the cochlea and auditory brain stem. Dr. Altschuler then joined the University of Michigan in 1985, at the Kresge Hearing Research Institute in the Department of Otolaryngology, with appointments in Cell and Development Biology and the Neurosciences Graduate Program.
Areas of Interest:
The Altschuler Laboratory studies the molecular and cellular elements that underlie mature auditory and vestibular function, identifies changes associated with disorders and develops mechanism based interventions for protection, treatment and repair. Current studies are on:
Noise-Induced and/or Age-Related Hearing Loss and Vestibular Disorder: Studies use mouse and rat models to study the progression of noise-induced or age-related changes in cellular elements, intracellular functional signaling pathways and cochlear sensory cells and their synaptic connections all leading to changes in physiological and behavioral metrics of auditory function
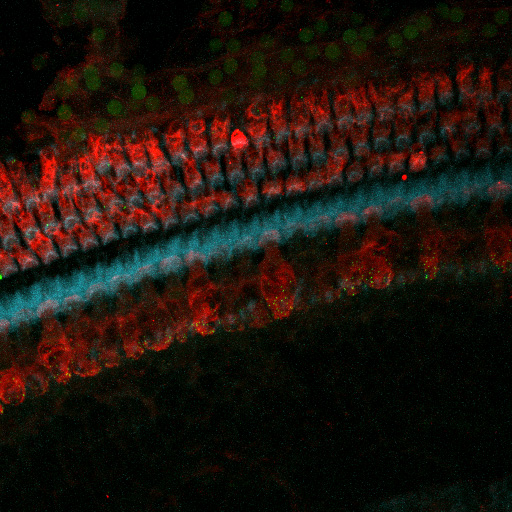
Noise-induced Tinnitus: Studies use the rat model to study noise-induced tinnitus, identifying and treating peripheral pathologies that could trigger the maladaptive central auditory changes associated with tinnitus.
- Protection and Repair for Noise-Induced Hearing Loss, Tinnitus and Age-Related Hearing Loss:
- One approach uses the knowledge we gain on mechanisms underlying each disorder to identify and test treatments. For example, we are testing treatments to prevent or repair noise-induced loss of inner hair cell – auditory nerve synapses to treat hearing loss and tinnitus.We are testing treatments targeting oxidative stress, ER stress and/or mTOR pathways to reduce or delay and age-related hearing loss.
- Another approach identifies endogenous protective mechanisms in the cochlea and develops interventions for their enhancement to increase protection and repair.
- A third approach is to develop use of stem cells (endogenous or transplanted) to replace or repair underlying pathologies.
RECENT PUBLICATIONS
- Altschuler RA, Dolan DF, Halsey K, Kanicki A, Deng N, Martin C, Eberle J, Kohrman D, Miller, RA, Schacht J (2015) Age-related Changes in Auditory Nerve – Inner Hair Cell Connections, Hair Cell Numbers, Auditory Brain Stem Response and Gap Detection in UM-HET4 Mice, Neuroscience 292:22-33
- Altschuler RA, Wys N, Prieskorn D, Martin C, DeRemer S, Bledsoe S, Miller JM, (2016) Treatment with Piribedil and Memantine Reduces Noise-Induced Loss of Inner Hair Cell Synaptic Ribbons, Sci. Rep. 6,
- Ross AM, Rahmani R, Prieskorn DM, Dischman AF, Wys N, Martin CA, Miller JM, Lahann L, Altschuler RA, (2016) Persistence, Distribution, and Impact of Distinctly Segmented Microparticles on Cochlear Health following In Vivo Infusion Journal of Biomedical Research Materials, 104(6):1510-22, 2016 PMID:26841263
- Stewart CS, Kanicki A, Altschuler RA, King WM, (2018) Vestibular short latency evoked potential (VsEP) is abolished by low frequency noise exposure in rats. J. Neurophys. 119(2):662-667


