Cochlear prostheses provide a safe and effective method to return hearing to the profoundly deaf. Cochlear prostheses work by directly stimulating the auditory nerve. However, there is often loss of auditory nerve with profound deafness, which can reduce the efficacy of cochlear prostheses. These studies are designed to enhance auditory nerve survival following deafness and to replace lost auditory nerve with stem cells in order to increase the efficacy of cochlear prostheses and to increase the candidate pool.
Auditory Nerve
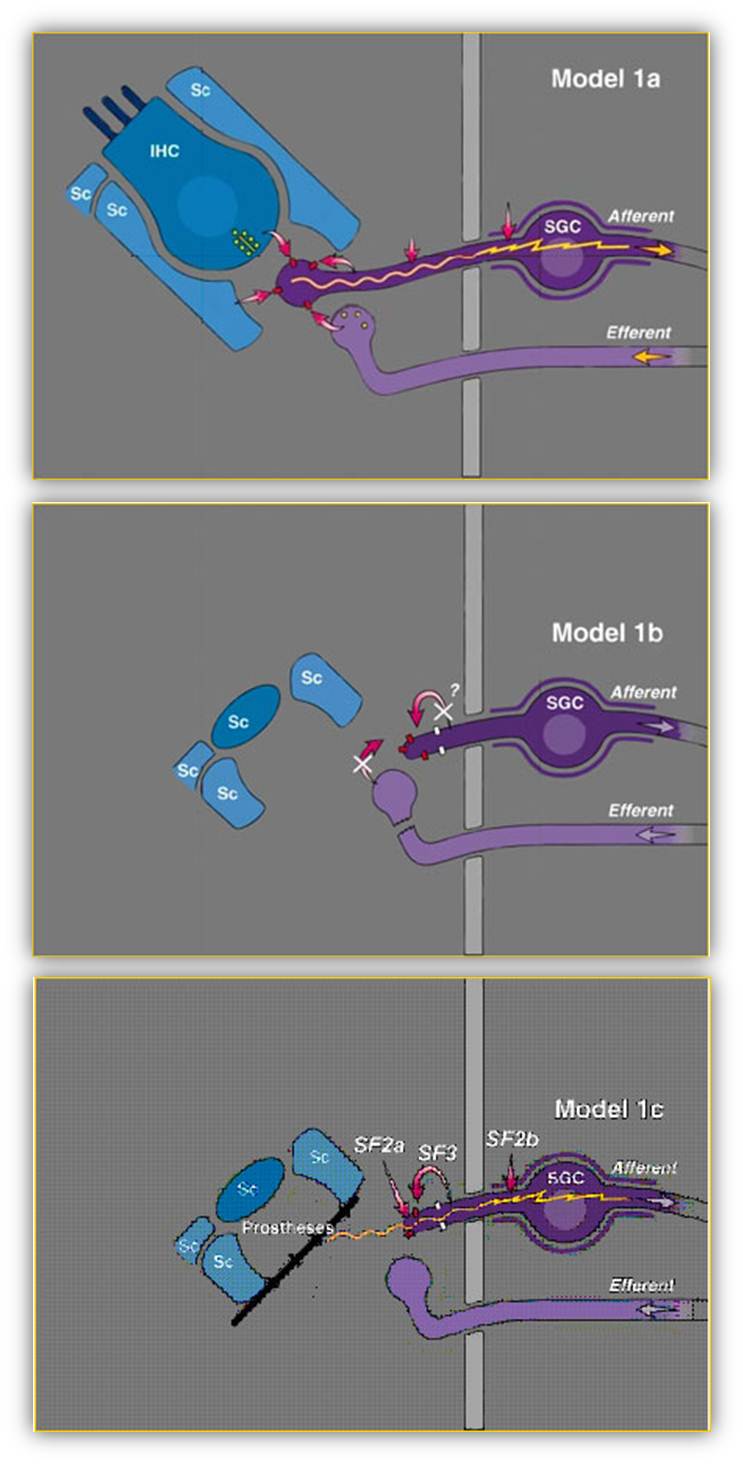
We are also studying regrowth of the peripheral processes of the auditory nerve, based on the model shown below. In this model, the normal connection between the inner hair cell and the auditory nerve is shown in Model 1a. There are multiple survival factors that provide support of the auditory nerve, many of these provided by the inner hair cell. When deafness caused a loss of inner hairs (Model 1b) many of these survival factors are lost as is the release of excitatory transmitter and resulting excitation of the auditory nerve. This contributes to a secondary loss of the auditory nerve. If some or all of these survival factors are provided to the deafened cochlea with inner hair cell, with neurotrophic factors provided through a pump and excitation by a cochlear prostheses (Model 1c) then auditory nerve survival is supported and the secondary loss is halted. Our studies are to develop these interventions to enhance auditory nerve survival following deafness. We have identified three types of interventions. Direct stimulation of the auditory nerve with a cochlear implant provides activity and significantly enhances survival. Application of neurotrophic "survival factors" such as BDNF, GDNF and FGF will also increase auditory nerve survival. These neurotrophic factors can be applied using mini-osmotic pumps with a cannula into scalar tympani. Dr. Yehoash Raphael has developed the use of gene transfer to apply neurotrophic factors to the cochlea. Application of antioxidants also enhances survival. Since these all act by different mechanisms, combinations of these interventions are more effective than if they are applied individually.
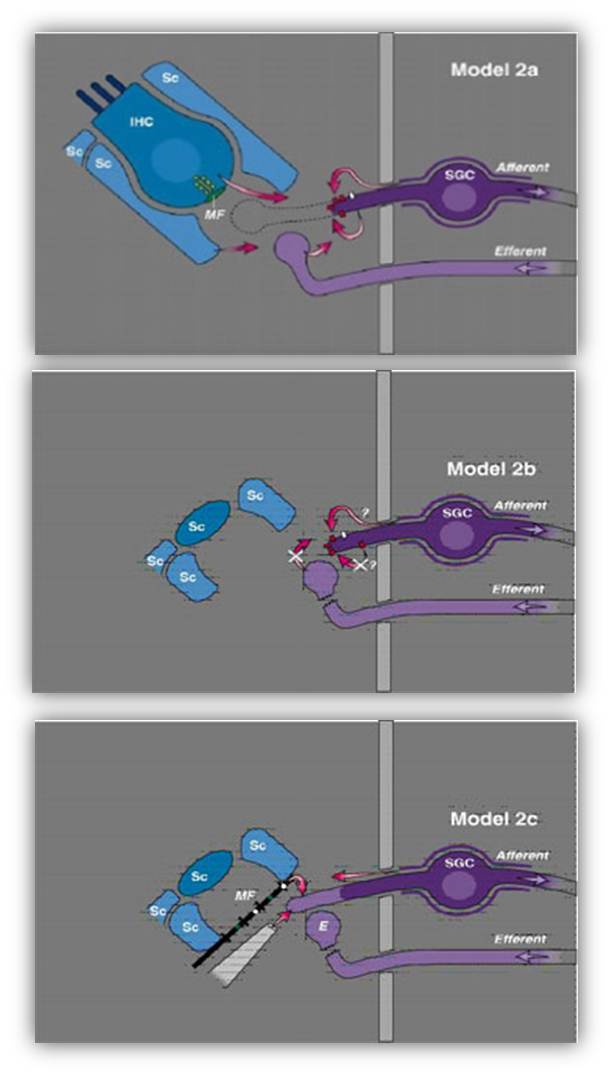
We find that neurotrophic factors such as BDNF, FGF and CNTF can induce the regrowth of the peripheral processes of the auditory nerve. This is demonstrated in Model Series #2, where Model 2a shows swelling and loss of the peripheral process of the auditory nerve, which cannot regrow when there is loss of inner hair cells (Model 2b), but application of exogenous neurotrophic factors will induce regrowth (Model 2c). Our studies are working to identify optimal interventions for inducing regrowth and methods to direct toward specific sites, perhaps on a cochlear prostheses.
Stem Cells
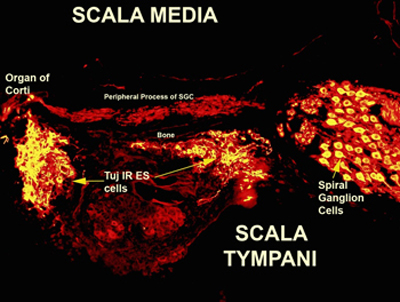
While the interventions described above can enhance auditory nerve survival following deafness, there still remains a significant patient population in which these interventions will be too late or ineffective. For these people there will be ongoing degeneration of the auditory nerve. This patient population then presents a powerful target for stem cell research, since cochlear prostheses, the treatment for profound deafness, depends on remaining auditory nerve for its function We are therefore collaborating with Joe Miller at KHRI and with Sue O'Shea, Kate Barald and Matt Velkey in the Department of Cell and Developmental Biology to develop the use of stem cells to replace lost auditory nerve, to allow and improve the function of cochlear prostheses. This is also an excellent model for stem cell neural regeneration in general, with the ability to easily assess and monitor return of function. Our studies show that mouse embryonic stem cells can be placed into guinea pig scala tympani where they survive and many differentiate into a neuronal phenotype (based on TuJ1 immunostaining) with many molecular features of spiral ganglion cells (auditory nerve neurons) including vesicular glutamate transporters.