Areas of Interest
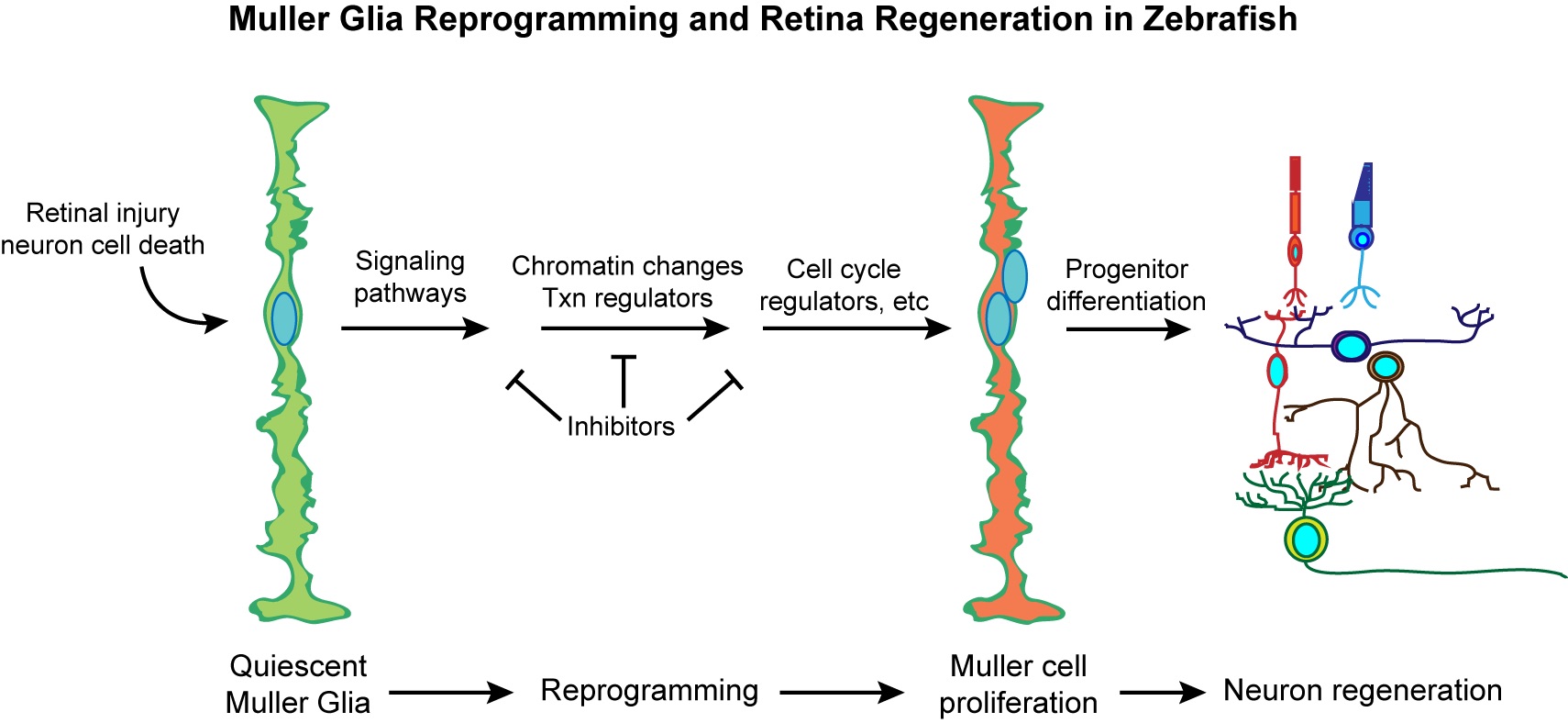
Research interests in the Goldman lab centers on cellular reprogramming and central nervous system (CNS) regeneration. We use both zebrafish and mice in our studies. Unlike mammals, zebrafish can regenerate their CNS. The retina is part of the CNS and we are using it as a model tissue for studying regeneration because of its relatively simple structure and its accessibility to experimental manipulation. The retinas of both fish and mammals contain 1 major glial cell type, named Muller glia. Normally Muller glial cells contribute to retina structure and homeostasis. However, in zebrafish, Muller glia also respond to retinal injury by undergoing a reprogramming event that endows them with properties of a retinal stem cell. In contrast, Muller glia in the mammalian retina respond to retinal injury by undergoing a gliotic response that is characterized by cell swelling and induction of intermediate filament proteins. The Goldman lab is researching the mechanisms by which zebrafish Muller glia reprogram and adopt stem cell properties to regenerate all major retinal neuron types. These mechanisms are then tested in mice to investigate if we can reprogram mammalian Muller glia to adopt stem cell properties similar to that seen in fish. It is anticipated that this kind of regenerative research will lead to new ways of treating blinding eye disease.
Honors & Awards
1994 University of Michigan, Research Scientist Recognition Award
1995 Mental Health Research Institute Discovery Award
2001 Wilson Scholar, Wilson Medical Research Foundation
2003 University of Michigan, Research Scientist Achievement Award
2010 Undergraduate Research Opportunity Program Recognition Award for Outstanding Research Mentorship
2013 Research to Prevent Blindness Innovative Ophthalmic Research Award
2014 Bernard W. Agranoff Collegiate Professor of Neuroscience, University of Michigan
2014 Elected AAAS Fellow, American Association for the Advancement of Science
2015-18 Interim Chair, Department of Biological Chemistry
Published Articles or Reviews
Tgfb3 collaborates with PP2A and notch signaling pathways to inhibit retina regeneration.
Lee MS, Wan J, Goldman D.
eLife, 2020; 9:e55137
Notch suppression collaborates with Ascl1 and Lin28 to unleash a regenerative response in fish retina, but not in mice.
Elsaeidi F, Macpherson P, Mills EA, Jui J, Flannery JG, Goldman D.
J Neurosci. 2018; 38(9):2246-2261
Opposing Actions of Fgf8a on Notch Signaling Distinguish Two Muller Glial Cell Populations that Contribute to Retina Growth and Regeneration.
Wan J, Goldman D.
Cell Reports 2017; 19:849-862.
Zebrafish Müller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons.
Powell C, Cornblath E, Elsaeidi F, Wan J, Goldman D.
Sci Rep. 2016; 6:24851.
Leptin and IL-6 Family Cytokines Synergize to Stimulate Müller Glia Reprogramming and Retina Regeneration
Zhao, X-F., Wan, J., Powell, C., Ramachandran, R., Myers Jr., M.G., Goldman, D.
Cell Reports 2014; 9:272-284.
Retinal Injury, Growth Factors, and Cytokines Converge on β-Catenin and pStat3 Signaling to Stimulate Retina Regeneration
Jin Wan, J., Zhao, X-F., Vojtek, J. and Goldman, D.
Cell Reports 2014; 9:285-297.
Müller glial cell reprogramming and retina regeneration.
Goldman, D.
Nat Rev Neurosci 2014; 15:431-442.
Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration.
Powell, C., Grant, A. R., Cornblath, E. and Goldman, D.
Proc Natl Acad Sci USA 2013; 110:19814-19819.
Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina.
Ramachandran, R., Zhao, X-F. and Goldman, D.
Nature Cell Biology 2012; 14:1013-1023.
HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration.
Wan, J., Ramachandran, R. and Goldman, D.
Dev Cell 2012; 22:334-347.
Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration.
Ramachandran, R., Zhao, X-F. and Goldman, D.
Proc. Natl. Acad. Sci. USA 2011; 108:15858-15863.
Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway.
Ramachandran R, Fausett BV, Goldman D.
Nature Cell Biol, 2010; 12:1101-1107.
Complete List of Published Work in My Bibliography:
http://www.ncbi.nlm.nih.gov/sites/myncbi/1Typvgk0kNLAu/bibliography/40444724/public/?sort=date&direction=ascending