
Paul Jenkins, Ph.D., Director, Jenkins Lab
Work in the Jenkins laboratory, led by Paul Jenkins, Ph.D., is focused on understanding the cellular and molecular mechanisms underlying psychiatric disorders, like bipolar disorder. Recent work from the laboratory gives important insight into the effects of ankyrin-G (ANK3) dysfunction on inhibitory signaling in the forebrain. Inhibitory GABAergic circuits are critical for the synchronization and higher order function of brain networks, and defects in this circuitry are linked to neuropsychiatric diseases, including bipolar disorder, schizophrenia, and autism.
Work in cultured neurons has shown that ankyrin-G plays a key role in the regulation of inhibitory synapses by interacting with the GABAA receptor associated protein (GABARAP). Recently, the Jenkins laboratory generated a knock-in mouse model in collaboration with Dr. Vann Bennett at Duke University that expresses a mutation that abolishes the ankyrin-G/GABARAP interaction (Ank3 W1989R). Ank3 W1989R mice exhibit a striking reduction in forebrain GABAergic synapses resulting in neural cell hyper-excitability and disruptions in network synchronization.
In collaboration with the Heinz C. Prechter Bipolar Research Program, the Jenkins lab identified the ANK3 W1989R variant in a family with bipolar disorder, suggesting a potential role of this variant in disease.
Our results highlight the importance of ankyrin-G in regulating forebrain circuitry and provide novel insights into how ANK3 loss-of-function variants may contribute to human disease. Current work is focused on how commonly-used therapeutics affect inhibitory signaling in the ANK3 loss-of-function mouse model, in an effort to identify novel therapeutic targets for the treatment of bipolar disorder. In addition, the lab is expanding its studies to include the examination of neurons generated from patient-derived stem cells to determine the effects of these variants in the exact genetic background of our patients.
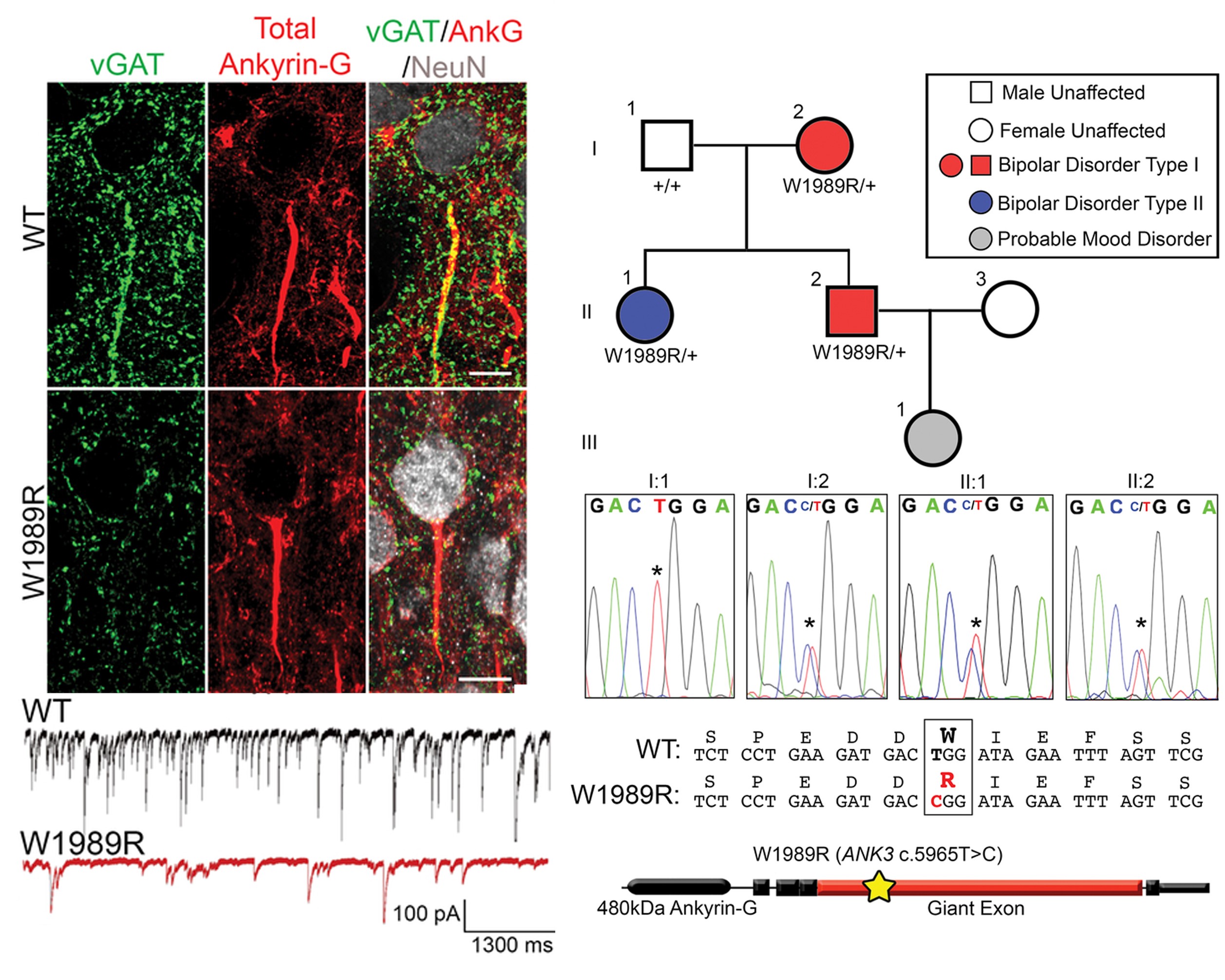
The ANK3 W1989R mutation causes loss of inhibitory synapses (left, green) on forebrain pyramidal neurons in mice. This causes decreases in inhibitory currents, the downward deflections in the electrophysiological recordings shown in this image. These currents are critical for normal forebrain synchronization and defects in these neurons have been implicated in a number of neuropsychiatric conditions, including bipolar disorder. The ANK3 W1989R variant is a rare variant present in 1 in 10,000 people of European American descent. This allele was found in a family participating in the Prechter Program. Four family members carry the ANK3 W1989R allele, all of which are affected by mood disorders.
