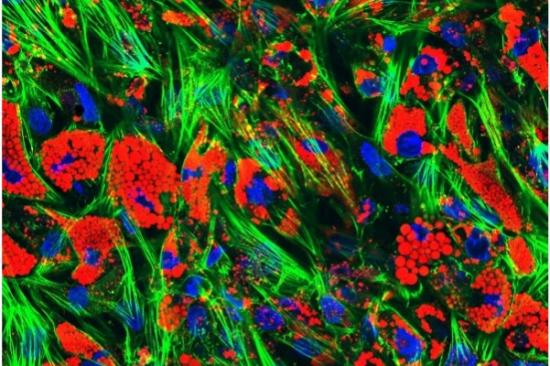
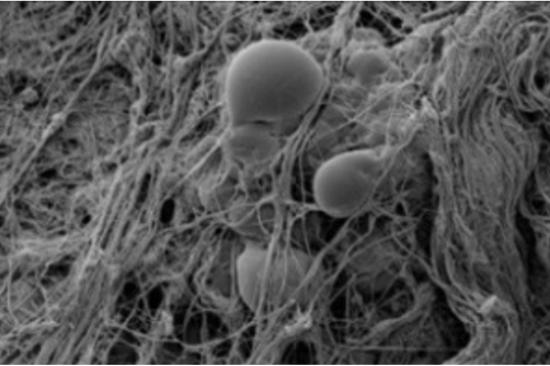
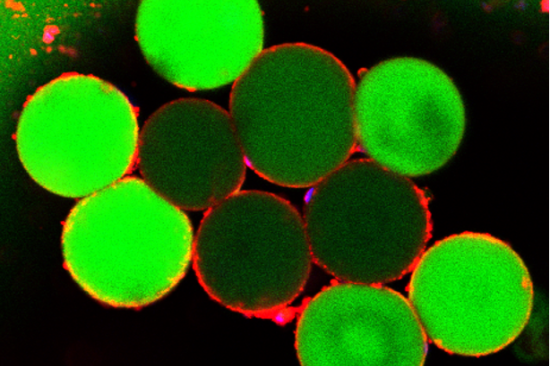
A major focus of the O'Rourke Lab centers on understanding the mechanisms involved in insulin resistance and adipocytes glucose metabolism. Our laboratory's unique adipose tissue resource, including a well-annotated human subcutaneous and visceral adipose tissue bank, enables our group to correlate our discoveries at the bench with patient physiology, histology and outcomes related to bariatric surgery, weight loss and disease remission. Our 2D and 3D human adipocyte cell culture models allow detailed mechanistic interrogation of these tissues.
We also use murine models to study the role of adipose tissue in regulating in vivo metabolism, along with models of adipocyte transplant to investigate the regulatory effects of adipocytes on metabolism and insulin resistance. A key objective of our work is to create therapeutic vehicles using engineered adipose tissue transplants to treat adipose tissue dysfunction in type 2 diabetes. We use advanced technologies, such as transcriptomics, RNA sequencing and single-cell sequencing, flow cytometry, and a range of cellular metabolic assays to answer questions about adipocyte dysfunction and its impact on systemic metabolism.