In 2004, Department of Surgery plastic surgeon Paul Cederna (M.D. 1989) got a call from Daryl Kipke, Ph.D. — a professor in the University of Michigan Department of Biomedical Engineering and a friend from when both were engineering undergrads at Michigan. Kipke was a scientist with a strong interest in brain probes and wondered about the ability of these probes to connect with peripheral nerves.
The military, said Kipke, was looking for proposals to enhance functional recovery following amputation. Was Cederna interested in working on it?
“He had a whole series of fancy brain probes he could use,” recalls Cederna, from his office in the Department of Surgery. “I did peripheral nerve work. I’d never worked in this area of prosthetic control, but I had an engineering background and I was really excited.”
A crack team was assembled — Cederna, Kipke, Melanie Urbanchek, Ph.D., David Martin, Ph.D., and a host of other colleagues from the U-M and other institutions — and a grant was written.
They got the grant. Here’s where things get a little … odd.
At the grant kickoff meeting in 2005, the team met with a project manager from the military to outline their plans for the $5.5 million they’d just received.
Suddenly, as Cederna recalls, the project manager slammed his fist on the table and shouted, “When are you people going to stop stuffing nails in nerves? I don’t want to hear another thing about nails in nerves!”
Silence. (For the record, no one had said anything about nails…)
“It was unbelievable,” says Cederna. “I asked, ‘Well, why did you just give us the grant?’ ”
“Because we believe in this team,” said the man. “Figure something out.”
In some ways, the project manager’s frustration was understandable. For years, researchers had been trying to figure out how to interface with the divided (cut) ends of peripheral nerves that simply end in an amputation stump. The brain continued to send impulses through the nerves to control a limb that was no longer present. There had to be a way to detect those impulses from the nerve and direct them into a prosthesis, to translate those impulses into vital actions — grasping a fork or a hairbrush, or holding a child’s hand. But it hadn’t proved simple.
Cederna explains. “For decades, researchers had wrapped cuffs around nerves, tried to get them to grow through tiny holes in disks, implanted probes into nerves … but over time, those devices would scar up, form painful neuromas, and eventually stop working. They weren’t stable.”
He recalls that it was a particularly somber and thoughtful lunch that day at the kickoff meeting, after the project manager’s outburst.
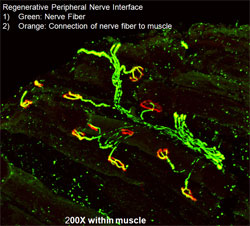
The team began pondering a new question: If a cut peripheral nerve doesn’t like being manhandled, what does it like? Says Cederna, “It occurred to me that we needed to address the biology of the divided peripheral nerve. We came up with the genesis of regenerative peripheral nerve interface (RPNI). No one had thought of that before.”
In RPNI, muscle tissue from the area near the nerve was placed around the nerve stump. A tiny electrode was embedded in the muscle. The team began testing the technique in rat models; the results were thrilling. The nerve grew naturally and easily into the muscle and reinnervated the muscle tissue. Time and time again, Cederna and his colleagues witnessed that signals from the brain came down the nerve and caused the newly innervated muscle surrounding it to contract.But there were unexpected bonuses. First, the muscle tissue actually amplified the signal coming from the nerve; even a faint impulse was easily detectable. Second, the nerve did not develop a painful neuroma.
The goal became clear: to get that signal — generated by the brain, and having traveled down the divided nerve and through the resected muscle and electrode — to communicate wirelessly with a prosthesis.
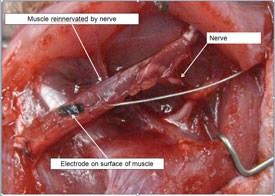
The RPNI is on track to revolutionize prosthetic technology and make life infinitely better for people who’ve lost limbs. And making life better for people — combined with the specialty’s inherent need for ever-shifting creativity — is what drove Cederna into plastic surgery in the first place.
Raised in Marquette, Michigan, Cederna, a high school athlete, was initially drawn to orthopedic surgery. After earning his bachelor’s degree in engineering from Michigan, he entered the Medical School, still confident in his goal. But in his third year at the U-M Medical School, a rotation in plastic surgery changed his course.
Within a day or two, he witnessed a breast cancer reconstruction and two complex facial reconstructions – working with tissue, tendons, blood vessels and bone.
“I was just amazed at the breadth of the cases they did, the creative solutions to solve these complex reconstructive challenges,” he says. “They had such depth of knowledge — they had to know everything about every area of the body.”
Today, as Cederna treats people with profound disfigurements due to burns, disease and other trauma, he’s continually reminded of the importance of the work he does. “We’re not cutting out cancer, but our work completely changes people’s lives,” he says.
Today, Cederna’s team — the one the military project manager so fervently believed in — is in the process of taking their research to the next step. In October, they implanted multiple RPNIs into the arm of a rhesus macaque monkey named Luther who is now being trained to voluntarily control an “avatar” — an image of a monkey arm on a computer screen. When a signal from Luther’s brain stimulates the RPNI, the onscreen avatar moves, and he gets a juice reward. When that’s mastered, the image of the arm will be replaced with an actual prosthetic arm, positioned on a table in front of him, that will receive signals, wirelessly, from the RPNIs in his arm. (Cederna jokes that once Luther masters control of the prosthetic arm, he could theoretically direct it to open his cage.)
“Our hope, our plan, is to be able to start a trial in humans within three years,” says Cederna. “We’re really excited about that as an option.”
He adds that the interface can go in both directions. Cederna sees a future in which prosthetic hands include sensory devices in the fingertips that can send signals back into a person’s residual limb through an RPNI and into the brain — signals so sensitive and accurate that the prosthetic limb could pick up a Styrofoam cup of hot coffee without spilling, or actually “feel” the silkiness of a baby’s head.
Ask Cederna about his most burning research question and the answer comes with a laugh: “How to get through the FDA faster!” But the next most-important thing to figure out, he says, is how to power his team’s new RPNI technology for the long haul. The simplest-seeming human action, like picking up a pencil, involves dozens of nerves and muscles and each RPNI device must be powered by an implanted source, similar to a pacemaker, that will somehow need to be regularly charged.
“We don’t need a solution that lasts two years — we need a solution for 60 years,” he says, explaining that most people living with limb amputations are young victims of trauma.
Cederna is quick to credit his entire team for the success thus far of this important project. That they’re not all at U-M (Kipke, for example, is now executive director of NeuroNexus, an Ann Arbor neurotechnology company) doesn’t hamper the research at all. Their brilliance, their determination and their spirit of collaboration are bringing new hope and new functionality to survivors of war, trauma and disease.
“It’s entirely possible that we’re getting there,” he says, “and it’s so exciting to see.”
Cederna stresses that the promise of the RPNI project is a team effort. He credits the researchers below for their significant roles.
Melanie Urbanchek, Ph.D., U-M Department of Surgery
Nicholas Langhals, Ph.D., U-M Department of Surgery
Daniel Aloi, Ph.D., chair of the Department of Electrical and Computer Engineering, Oakland University
Brent Gillespie, Ph.D., U-M Department of Mechanical Engineering
Greg Gerling, Ph.D., Department of Systems and Information Engineering, University of Virginia
Steve Goldstein, Ph.D., professor emeritus, U-M Departments of Mechanical Engineering, Biomedical Engineering, Orthopaedic Surgery
Daryl Kipke, Ph.D., executive director, NeuroNexus, Ann Arbor
David Martin, Ph.D., the Karl W. and Renate Boer Professor and chair of the Department of Material Engineering, University of Delaware
Asamah Rawashdah, Ph.D., P.E., Department of Electrical and Computer Engineering, Oakland University


