Then and Now: Always Searching
1890s - 1930s
1896
George Dock publishes Some Notes on the Coronary Arteries, in which he diagnoses a heart attack in a living patient and describes its pathophysiology.
1916

Louis Harry Newburgh establishes internal medicine’s role as a leader in metabolism and nutrition through rigorous measures of energy balance that requires subjects to spend days in a calorimetry chamber, precisely measuring their inputs and outputs. Over the next 36 years, Newburgh will demonstrate that obesity is caused by more energy flowing in through diet than out, and that weight loss can reverse glucose intolerance in what we now call type 2 diabetes. Before the availability of insulin, he will show that a high-fat, low-carbohydrate and -protein diet can control type 1 diabetes. He will also use diet to control edema in chronic nephritis patients.
1920

Frank Norman Wilson begins a 32-year career at U-M, where he will become a world leader in electrocardiography. By focusing on the tracings’ form, rather than rhythm, he will be the first to properly identify ECG patterns in bundle-branch block. While seeking a mechanism for acquiring unipolar, semidirect recordings, in 1934 he will develop the central terminal, which will give rise to the six precordial leads and form the basis of the modern ECG machine. He will establish conventions for depicting polarity and placing leads. And his analysis of the QRS complex will explain how heart attacks produce Q waves and how their patterns can reveal distribution of injury.
1927

Through a philanthropic gift, the Thomas Henry Simpson Institute for Medical Research is launched under Cyrus Cressey Sturgis, also chair of internal medicine. Founded to study pernicious anemia, the institute develops ventriculin, a treatment derived from hog stomach that gives patients an alternative to ingesting a half-pound of liver daily. It will be developed through an innovative partnership between U-M and Parke-Davis. As pernicious anemia becomes treatable, the institute broadens its focus to general hematology.
1937
The Rackham Arthritis Research Unit is established as one of the first arthritis research units in the U.S. Its early research in areas such as rheumatoid arthritis, gout, and the biology of synovial tissue will lay the groundwork for multiple NIH grants from 1977 onward.
1940s - 1960s
1948

James Neel is named director of U-M’s Heredity Clinic. Over a 39-year career at U-M, Neel will develop a reputation as a “father of modern human genetics.” He will identify the genetic basis for sickle cell anemia, study chromosomal damage from atomic radiation and viruses, and propose the “thrifty gene” hypothesis, suggesting that genes associated with diabetes and obesity remain in the gene pool because they protected our ancestors in times of deprivation. He will also establish the first academic department of human genetics in the U.S., which he will chair for a quarter-century.
1954

Jerome Conn describes primary aldosteronism, later named Conn’s Syndrome, a curable form of high blood pressure. Caused by an adrenal tumor secreting too much aldosterone, the disorder is one of the few serious forms of high blood pressure that can be cured by surgical removal of the tumor if recognized early. Conn will go on to devise early-detection techniques for adrenal tumors, and U-M will become a worldwide center for the study and treatment of this and other adrenal conditions.
1954
Kenneth Matthews begins exploring the immunologic mechanisms underlying urticaria and will help clarify the role of IgE in this process. His seminal findings help to define “hives” as an allergic phenomenon rather than a neurological one.
1957
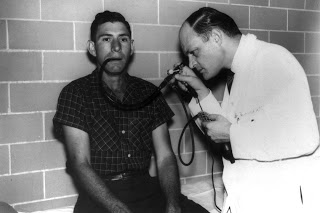
Basil Hirschowitz leads a U-M team that creates the first flexible fiber optic endoscope, which he tests by swallowing the prototype. The scope will become the standard tool for visualizing the GI tract, revolutionizing diagnosis and treatment. The original instrument will later be housed at the Smithsonian.
1962

R.J. Bolt and Arthur French develop a simplified, multiple-retrieving small-bowel biopsy tube that allows them to perform the first intestinal biopsy and to show the relationship between flattened villi and malabsorption in celiac disease.
1964

Stefan Fajans describes Maturity-Onset Diabetes of the Young, a subtype of type 2 diabetes that appears in young people because of a defect in a single dominant gene. He did this by studying a large Michigan family where 74 members inherited the condition. Through this, Fajans helps establish that diabetes is not a single disease, but consists of several subtypes with different causes. Fajans will remain engaged with the division for more than 65 years and will later co-identify a genetic marker for MODY, as well as the gene itself.
1965
The State of Michigan advances gerontology as a field of inquiry in the U.S. by creating the Institute of Gerontology at U-M, the nation's first state-funded center on aging. Though it begins with a broad exploration of aging in society, it will evolve to focus increasingly on the basic science of aging until being merged with the Geriatrics Center in 2004.
1969
The Michigan Kidney Registry is founded by Ron Easterling with a grant from the National Kidney Foundation. It is the first statewide, end-stage renal disease registry in the U.S., charged with collecting and analyzing patient data to determine how best to treat end-stage kidney disease. In 1981, the registry will produce a seminal study showing the superiority of transplant to dialysis. As the first to address time-to-treatment bias, the study proves instrumental in convincing clinicians to more aggressively pursue transplant for all candidates.
1970s
1971

William Beierwaltes (MEND & Nuclear Med) drives the development of the first radiopharmaceuticals capable of imaging the adrenals. Working with U-M radiochemists, he helps develop 131-I-radiocholesterol — the basis for later agent NP-59 — for functional imaging of the adrenal cortex, as well as metaiodobenzylguanidine (MIBG) for imaging medullary tumors. These agents will radically transform the diagnosis of pheochromocytoma and neuroblastoma, distinguish different forms of Cushing’s and Conn’s syndromes, and differentiate benign from malignant adrenal cortical tumors.
1971

C. William Castor (Rheum) starts to identify the inflammatory mechanisms responsible for joint destruction in rheumatoid arthritis. By comparing fibroblasts taken from the joints of RA patients to normal controls, he not only characterizes the cells’ differences in metabolism and proliferation, but also identifies cell and chemical stimuli that can induce RA characteristics in normal fibroblasts. His decades-long contribution will receive rheumatology’s highest honors, and his research thread will be picked up in the ’90s by David Fox (Rheum), who will reveal how fibroblasts of the joint interact directly with lymphocytes in RA.
1971

Stevo Julius conducts seminal research on prehypertension, showing an increase of sympathetic tone in this very early stage of hypertension and that chemical blockade of autonomic nerves normalizes blood pressure in some patients. He will use the population-based Tecumseh Study to demonstrate the association of prehypertension with cardiac and vascular damage, and the multicenter TROPHY trial to show that early treatment can postpone, but not prevent, later hypertension.
“Over his 40-year career at U-M, Stevo Julius changed the way physicians understand and treat hypertension. His work truly bent the curve in reducing mortality from cardiovascular disease.”— David Pinsky
1976

William Solomon launches seminal studies on the allergenicity of airborne particles smaller than pollen grains. He will help identify the prevalence patterns of these aerosols involving various allergens, suggest techniques for minimizing exposure and reveal how chronic exposure actually induces allergy by allowing these small allergens to penetrate the epithelium of the nose and lungs.
1977

Robert Fekety implicates a Clostridial toxin in severe antibiotic - associated diarrhea, helping to establish Clostridium difficile as the infectious agent responsible for this potentially fatal colitis. He also publishes guidelines for the diagnosis and management of C. diff, and will go on to clarify its method of transmission, epidemiology, risks, and complications.
1977
Having identified 100 researchers with work relevant to diabetes, U-M is one of five U.S. institutions to be awarded an NIH-funded Diabetes Research and Training Center. Under the direction of Stefan Fajans, it focuses on basic cell regulation as well as the natural history, genetics, management, and treatment of diabetes.
1978
U-M launches the Clinical Research Center under Irving Fox. A precursor to MICHR, the center will facilitate the development of new treatments for Wilson’s Disease as well as Fox’s own identification of the relationship between the antirejection drug cyclosporine and the development of gout in kidney transplant patients.
1980s
1980
PCCM researchers led by David Danztker and Jack Weg conduct cutting-edge large-animal and human research that reveals mechanisms of impaired oxygen uptake in acute respiratory distress syndrome (ARDS). In the next three decades, multi-investigator programs headed by Robert Strieter and Theodore Standiford will improve our understanding of ARDS by clarifying the role of host factors and progenitor cells in lung injury. PCCM researchers led by Galen Toews and Robert Hyzy will participate in landmark clinical trials identifying low tidal-volume ventilation as the standard of care in ARDS and showing the importance of reducing intravascular volume to reduce fluid accumulation in the lung.
1982
William Kelley and James Wilson advance our understanding of the biology and genetics of gout by publishing the complete amino acid sequence of HGPRT, the enzyme whose deficiency Kelley identified as the cause of Lesch-Nyhan syndrome and early-onset gout (Kelley-Seegmiller syndrome). Lab members will go on to sequence several mutant forms of HGPRT and, with Thomas Pallela (Rheum) and collaborators, to successfully transfect the human HGPRT gene into neuronal cells in culture and intact animals. Based on this technique, Kelley and colleagues will later secure a patent for in vivo gene therapy using viral vectors that will be the first and broadest to be issued in the field of gene therapy.
1983
U-M is recognized for its expertise in diabetes complications by becoming one of three national data sites for the NIH Diabetes Control and Complications Trial. In it, researchers help show that intensive insulin therapy significantly reduces eye, kidney and nerve complications in type 1 diabetes. In 1997, U-M researchers will show that tight blood glucose control in type 2 diabetes can also substantially reduce complications. Still later, in the Epidemiology of Diabetes Interventions and Complications study, MEND’s Rodica Pop-Busui will show that tight glycemic control in type 1 diabetes also protects against cardiovascular autonomic neuropathy.
1984

Francis Collins joins the U-M faculty. During his time at U-M, he will, with Michael Iannuzzi (PCCM), identify the cystic fibrosis gene by novel chromosome walking and jumping techniques, and, with James Wilson (MMG), go on to correct cystic fibrosis defects in patient-derived cells in a model system using gene transfer approaches. He will later discover the genes responsible for neurofibromatosis and will help identify those for Huntington’s disease. In 1993, Collins will join the NIH to lead America’s Human Genome Project, which will map the entire human genome by the year 2000.
1984

Fred Morady (CVM), who previously performed the first cardiac ablation in man, arrives at U-M and quickly establishes it as an internationally recognized electrophysiology research center. Morady and Hakan Oral (CVM) will optimize the ablation technique, develop new catheters, and demonstrate that atrial fibrillation can be generated not only in the atrium itself but by tissue extending along the pulmonary veins.
1985

David Ginsburg
David Ginsburg (Hem/Onc & MMG) joins the faculty. Over the next three decades, his lab will help unravel the molecular mechanisms underlying several important bleeding and clotting disorders, including von Willebrand disease, thrombotic thrombocytopenia purpura (TTP), and combined factor V and factor VIII deficiency.
1986

Tachi Yamada and Chung Owyang (GI) establish the Michigan Gastrointestinal Peptide Research Center, the only center in the nation devoted to the study of GI peptides in the pathophysiology, diagnosis, and treatment of GI disorders. The Owyang lab will discover that somatostatin both excites and inhibits myenteric cholinergic transmission and plays a crucial role in mediating both limbs of the peristaltic reflex. This will lead to the use of somatostatin to treat bowel bacterial overgrowth in patients with chronic intestinal pseudo-obstruction. It is the first instance where a gut peptide is used to treat a GI disorder.
1987

Josephine Briggs (Neph) establishes how the kidneys autoregulate. She details the feedback loop that responds to sodium concentrations in the blood by adjusting filtration rates — articulating for the first time how the macula densa and enzyme renin regulate blood flow to the nephrons. Briggs will later head the National Institute of Diabetes and Digestive and Kidney Diseases’ Division of Kidney, Urologic and Hematologic Diseases.
1987

With support from the John A. Hartford Foundation, UMHS launches a U-M regent-designated multidisciplinary Geriatric Center. Led by Jeffrey Halter (GPM), it is one of the first such centers in the U.S. to focus on education, clinical care, and research related to the health of older adults, and will form the basis for major national grants just two years later.
1988

W. Joseph McCune (Rheum) develops a new treatment protocol for patients with severe lupus. He demonstrates that monthly intravenous infusions of cyclophosphamide can protect kidney function and save the lives of patients whose disease is worsening despite high-dose corticosteroids. Tens of thousands of lupus patients will be treated around the world with this protocol.
1989

Sally Camper (MMG) establishes the Transgenic Animal Core Facility, which will soon include embryonic stem cell technology. Using transgenic mice, Camper will identify a gene responsible for congenital deafness in humans and mice by showing that mutations in myosin 15 lead to defects in hair cells of the inner ear.
1989
Craig Thompson (Hem/Onc) shows that stimulating the CD28 pathway activates T-cells, prompting them to produce multiple lymphokines/cytokines. This pathway will be foundational to Thompson’s post-U-M career, during which he will clarify CD28’s role in T-cell apoptosis, immune costimulation, and glucose metabolism. His insights will stimulate new ways of treating autoimmune disease by blocking CD28 signaling, of inducing apoptosis as a therapy for lymphoma/leukemia, and of targeting cancer by interrupting its metabolism.
1989
An indication of its rapid ascent in geriatrics, U-M is awarded the region’s first VA Geriatric Research, Education, and Clinical Center (GRECC) and the National Institute on Aging’s first Geriatrics Center (later renamed the Claude D. Pepper Older Americans Independence Center). Initially under the leadership of Jeffrey Halter, the GRECC will later shift to Neil Alexander and the Geriatrics Center to Raymond Yung. Both centers feature an array of multidisciplinary research, such as groundbreaking mobility studies by Alexander and collaborators in engineering and neurology that will shed light on the mechanisms, measurement, and treatment of biomechanical changes related to aging and age-related disease.
1989

Roger Wiggins and colleagues are awarded one of the first George M. O’Brien Kidney Centers, an NIH program established by Congress to promote state-of-the-art basic and clinical kidney research to improve the lives of patients with kidney diseases. The U-M Kidney Center has remained continuously funded by the NIH since that time.
1989

Elizabeth Nabel and Gary Nabel demonstrate the ability to transfer and express genes within endothelial cells of the arterial wall in an animal model, highlighting the technique’s potential in cardiovascular disease. They will also show that fibroblast growth factor-1 can stimulate new blood vessel growth and may have potential for improving blood flow in selected clinical settings. After U-M, Elizabeth will direct the National Heart, Lung and Blood Institute and Gary, vaccine research at the National Institute of Allergy and Infectious Diseases.
1990s
1990
U-M is one of only four U.S. institutions designated as a Human Genome Research Center to map the human genetic blueprint.
1991

Fernando Martinez and Galen Toews begin to build a leading research program in idiopathic pulmonary fibrosis. In the decades to follow, the Pulmonary and Critical Care Medicine researchers, including Kevin Flaherty, Bethany Moore, and Marc Peters-Golden, will help identify key cells and mediators involved in fibrotic lung disease; test new IPF drugs; and contribute to enhanced diagnostics, such as demonstrating that the presence of fibroblastic foci in the lung tissue of IPF patients is predictive of the disease’s clinical course.
1991
Stephen Emerson combines molecular and cell biologic insights with principles of chemical engineering to develop the first stem cell bioreactor for the production of bone marrow stem cells and blood cells. He also identifies bone-forming cells (osteoblasts) as the key cell in bone marrow that directs blood cell production. These two programs will help advance the science of tissue engineering and our understanding of the role of microenvironmental cues in normal and neoplastic development.
1992

In two separate experiments just days apart, gene therapy is used by Gary Nabel to fight advanced melanoma and by James Wilson (MMG) to fight familial hypercholesterolemia, an inherited liver disorder causing extremely high levels of blood cholesterol. This is the first gene therapy ever performed in the Western world outside the National Institutes of Health.
1993
Andrew Feinberg helps reveal the role of imprinted genes, in which only one parent contributes a working copy, and loss of imprinting in cancer. He clarifies the link between DNA methylation and cancer, and establishes the molecular basis of the cancer-predisposing disorder Beckwith-Wiedemann syndrome. This work helps establish the causal role of epigenetics — changes in gene expression that do not involve changes to the underlying DNA sequence — in human cancer.
1995
Rick Boland uncovers the role of DNA mismatch repair in regulating the G2/s cell cycle checkpoint, and accurately predicts that colon cancer with microsatellite instability will be resistant to chemotherapy.
1995

Hepatologist Anna Lok is recruited to U-M. Over the next 20 years, her research will form the basis for international guidelines on the diagnosis, prevention, and treatment of hepatitis B. She will conduct seminal work on the natural history of hepatitis B and C and the role of hepatitis B virus genotypes and variants in patient outcomes. She will also lead the first study showing that hepatitis C can be cured with a combination of oral direct-acting antiviral agents without the need for interferon.
1996
Rodney Hayward is named director of the VA Health Services Research and Development Field Program, which later evolves into the Ann Arbor VA Center for Clinical Management Research (CCMR) under the leadership of Eve Kerr. The program grows from a small group of investigators with two VA-funded projects to an elite health services research center with over 40 investigators, 150 staff, and annual extramural funding of more than $18 million. Center investigators go on to do groundbreaking work using clinical information about the risk and benefit of medical services for individual patients to develop and test better models for decision support and performance measurement.
1996
Kim Eagle and colleagues form the International Registry of Acute Aortic Dissections, which will go on to enroll more than 6,500 patients from 42 aortic centers in 13 countries. Through nearly 100 publications, Eagle and fellow IRAD investigators will rewrite our understanding of how this highly lethal condition presents and is managed, and will elucidate multiple opportunities to improve care. For example, IRAD will show that urgent surgery, even in very high-risk patients who would previously have been denied an operation, can be life-saving, both immediately and in the long term.
1997
With more than 25 years’ experience running the Michigan Kidney Registry, U-M teams up with the Urban Institute in Washington, D.C., on a successful bid to coordinate the first national kidney registry, the United States Renal Data System. The team, under Friedrich Port, will lead the collection and analysis of data on chronic kidney and end-stage renal disease in the U.S. for more than a decade.
1997
Thanks to extensive drug development by George Brewer, the FDA approves zinc acetate as a maintenance therapy for Wilson disease, a potentially fatal genetic condition in which the liver cannot remove excess copper. His group’s studies of dosage, drug interactions, and mechanism of action will lead to zinc becoming the treatment of choice for both its efficacy and improved side effect profile.
1997

Dean Brenner demonstrates how to use tissue-based biomarkers to test cancer preventive compounds, a particular intellectual and methodological challenge when the endpoint — the development of cancer — could be decades away. In a paper confirming that aspirin hits its target in the colon, Brenner outlines how to determine a compound’s efficacy, optimal dosing, and safety. Over the next 25 years, he and colleagues will develop a robust biomarker validation system and will use molecular and stem cell-based biomarkers to test numerous natural cancer preventives such as turmeric, ginger, and pepper.
1997

Stephen J. Weiss is named editor-in-chief of the Journal of Clinical Investigation. His lab is involved in identifying key molecules leading to cancer growth and metastasis. Termed Snail1 and MT1-MMP, these factors control normal growth and movement of cells during embryonic development and postnatal development, but also act as master switches in cancer, where their inappropriate reactivation leads to abnormal growth, invasion, and metastasis. The lab will later explore their value as therapeutic targets.
1998
James Baker is named director of a new center that will become the Michigan Nanotechnology Institute for Medicine and Biological Sciences, which aims to help move promising biological nanotechnologies to market. Baker’s own work within the institute focuses on the development of nanoemulsions, including topical antimicrobials for the military and a new type of effective, non-livevirus nasal vaccine. The vaccine, which will later be acquired by a pharmaceutical company, is effective on a variety of mucosal surfaces, making it a platform delivery system suitable for organisms ranging from inhalational anthrax to herpes to hepatitis B.
1999


Adrenal cancer expert Gary Hammer joins the MEND Division. Studying adrenal growth and development, he will become central to the discovery of adrenal stem cells and how associated genetic defects lead to diseases of adrenal failure and adrenal cancer. Building on the international adrenal network that he and David E. Schteingart ignited, he will later help to identify and leverage critical genetic drivers of adrenal cancer to launch newly developed targeted therapies for this rare and deadly disease.
1999
Mark Fendrick develops the concept of value-based insurance design, which aims to increase consumer adherence with recommended care guidelines by aligning consumer out-of-pocket costs with the potential clinical benefit of certain health services and medications. V-BID will later be included in the Affordable Care Act.
1999
As PI of the RALES trial, Bertram Pitt improves survival in patients with heart failure by showing that adding a mineralocorticoid receptor antagonist to standard therapy reduces mortality and hospitalization in patients with chronic severe heart failure. He will contribute additional insights through the later EPHESUS trial.
2000s
2000

Peter Ubel heads the Center for Behavioral and Decision Sciences, which will become the Center for Bioethics and Social Sciences in Medicine. This multidisciplinary center conducts research, education, and public outreach on medical and health issues in society, such as ethics, justice, genomics, communication, and decision-making.
2000

Eric Fearon defines the role of the adenomatous polyposis coli tumor suppressor protein in the regulation of the beta-catenin and gamma-catenin proteins in colorectal tumor development. He will later show how the p53 tumor suppressor gene activates the miR-34 microRNA family in normal cells and how p53 loss of function in cancer cells leads to an inability to induce miR-34, resulting in enhanced cancer cell growth and a failure of the cancer cells to undergo programmed cell death.
2002
U-M’s Life Sciences Institute (LSI) is founded under Alan Saltiel. He will recruit some 450 faculty and staff, including five HHMI investigators, two NAS members, five NAM members, seven Pew and Searle awardees, and two MacArthur awardees. He will also establish a $220 million LSI endowment and launch centers for stem cell biology, chemical genomics, structural biology and medicine discovery, as well as the U-M-Israeli Partnership for Research.
2002

Daniel Clauw shows a biological basis for augmented pain processing in fibromyalgia through imaging studies and, later, neurochemical assays.
2003

Max Wicha is the first to identify cancer stem cells in a solid tumor. He and Hem/Onc colleagues will go on to clarify the molecular underpinnings of stem cell behavior, showing that mutations in HER2 (human epidermal growth factor receptor 2) and PTEN (protein coding) genes trigger rapid stem cell division and self-renewal in breast cancer, causing them to invade surrounding breast tissue. They will also conduct the world's first clinical trial of a treatment, called MK-0752, targeting breast cancer stem cells.
2003

The FDA approves a radiolabeled monoclonal antibody developed by Mark Kaminski for the treatment of non-Hodgkin’s lymphoma. The new agent, 131 tositumomab (Bexxar), uses cancer cell-seeking antibodies tagged with a radioisotope to target radiation directly to tumors while minimizing exposure to normal cells. The drug yields response rates up to 95 percent, including complete, multiyear remissions, even in patients no longer responding to chemotherapy. This work helps further the paradigm of molecularly targeted cancer therapeutics.
“Mark Kaminski is one of the select few who translated a basic science concept into mainstream clinical care. That is an enormous accomplishment.” — Dean Brenner
2003

Hepatologist Robert J. Fontana launches the Michigan Hepatotoxicity Clinical Research Network as one of six NIH-sponsored groups across the nation studying drug-induced liver injury. Hoping ultimately to identify genetic associations with DILI, the network’s early findings alert physicians to the higher than expected incidence of DILI, key risk factors, new causality assessment methods, and the range of drugs and herbal and dietary supplements implicated in liver injury in American patients.
2004
Daniel F. Hayes reports that circulating tumor cells are prognostic in metastatic breast cancer, paving the way for worldwide research into phenotypic and genotypic characterization of CTCs to better understand the metastatic process and to evaluate and monitor patients with breast and other types of cancer. He will go on to demonstrate the value of other cancer markers, and will become a leader in developing criteria and guidelines for generating and validating tumor biomarker tests for clinical use.
2004
Cem Akin launches the mastocytosis program, one of the few around the world dedicated to studying and treating this puzzling allergy-like disorder. He will go on to expand his work on mutations in the tyrosine kinases that allow mast cells to overproduce and will demonstrate the potential of TK-inhibitors to treat the disease, paving the way for clinical trials. He will also describe the first diagnostic criteria and classification for mast cell activation disorders, which are then internationally adopted.
2005

Juanita Merchant clarifies the mechanisms by which Helicobacter pylori infection causes stomach ulcers and cancer, showing that cytokines released after infection both stimulate gastrin production and prevent its inhibition, increasing stomach acid and causing inflammation. She will later implicate the Hedgehog pathway in the transition from inflammation to cancer, and identify a potential biomarker of this transition.
2005

The U-M Center for Stem Cell Biology is established. Under the direction of Sean Morrison, the center will take a leadership role in educating the Michigan public during the successful 2008 ballot initiative to protect and regulate human embryonic stem cell research in the state constitution. Morrison’s own lab will help clarify the molecular mechanisms that regulate the maintenance of adult stem cells. Their work reveals that the regenerative capacity of our tissues declines during aging due to increased expression of tumor suppressor genes in stem cells that inhibit the development of cancer.
2005
Stephen Gruber highlights the potential protective effect of statins in colorectal cancer development and will later demonstrate the protective effect of hormone replacement therapy for colorectal cancer in post-menopausal women.
2006

Charles Burant launches the Michigan Metabolomics and Obesity Center, which will evolve to house two NIH-funded centers, the Nutrition Obesity Research Center and the Regional Comprehensive Metabolomics Resource Core. The latter is one of only six metabolomics cores that support the nation’s research community in investigations in cancer, aging, metabolic diseases, and the microbiome. Burant’s own work helps to define the mechanisms by which genetically determined, intrinsic oxidative capacity is related to a reduced risk of diabetes and obesity and is associated with extended lifespan.
2006

Vallerie McLaughlin becomes the PI for the Data Coordinating Center of the Pulmonary Hypertension Breakthrough Initiative. Under McLaughlin, U-M maintains the clinical data for a consortium of 13 institutions biobanking lung and blood samples from pulmonary hypertension patients undergoing transplant. The goal is to use pathology, proteomics and genomics to understand and treat this devastating, progressive disease. In 2015, McLaughlin will co-publish findings from a phase 3 clinical trial showing positive results from the first prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension.
2007
The A. Alfred Taubman Medical Research Institute is launched to help physician-scientists speed the development of effective disease treatment. Housed in the A. Alfred Taubman Biomedical Science Research Building, its activities include the development of stem cell lines and a Taubman Scholars program with three-year grants for leading investigators, many from U-M Internal Medicine, working on issues such as cancer, cardiovascular disease, diabetes, and obesity.
2007

Bruce Richardson establishes the importance of epigenetics in autoimmunity by revealing how lupus-causing drugs can change gene expression in normal lymphocytes, prompting them to attack the body’s own cells. He will also show that diet and environmental agents that cause oxidative stress trigger lupus through similar changes in gene expression.
2007

Alan Saltiel discovers a switch in the activation of the innate immune system in obesity, resulting in a more inflammatory state in fat tissue and directly leading to insulin resistance and diabetes. He will go on to elucidate the immune pathway that is activated, and discover a drug that prevents this process. The latter will be investigated for the treatment of obese patients with type 2 diabetes.
2008
José Jalife, who leads a world-renowned basic and translational arrhythmia research group, establishes the U-M Center for Arrhythmia Research and is later joined by Héctor Valdivia, an expert in the study of calcium homeostasis. They will work together to clarify the role of ion channels in the genesis of arrhythmia and to identify the tornado-like rotating electrical waves that characterize atrial fibrillation. They will also work with the division’s clinical arrhythmia researchers to better map irritable areas of the heart and target them for treatment.
2008
The George M. O'Brien Kidney Center is reimagined as a core center to support clinicians worldwide in kidney disease research. The center, which will come under the leadership of Frank Brosius in 2010, includes a biobank with a representative cohort of chronic kidney disease patients across the U.S. and provides support in areas such as systems biology, bioinformatics, and transgenic animal models. Using the center’s infrastructure, teams led by Matthias Kretzler and Frank Brosius will discover that increased activation of the JAK/STAT signaling pathway in diabetes is a primary cause of kidney scarring and dysfunction, leading to successful clinical trials of a JAKII inhibitor. They will also use the center to identify the first-ever chronic kidney disease biomarker, by showing that low levels of epidermal growth factor in urine can identify patients at risk for end-stage kidney disease.
2008

Kenneth Jamerson uses the ACCOMPLISH study to challenge current hypertension treatment guidelines by showing that a fixed-dose combination therapy of a calcium-channel blocker with an ACE inhibitor leads to superior cardiovascular outcomes compared to current recommended diuretic-based therapies. Jamerson is also a leader in using innovative methodologies to research cardiovascular disease in the African- American community, with the goal of devising better treatment strategies.
2009
U-M purchases the former Pfizer campus and begins converting it into the North Campus Research Complex, a hub for collaboration-minded scientists across U-M interested in “igniting improvements to humanity’s health and well-being.” It will offer resources ranging from scientific support to technology transfer, and will house interdisciplinary programs with heavy Internal Medicine representation, such as the Institute for Healthcare Policy and Innovation, Translational Oncology Program, Center for Arrhythmia Research, Center for Health Communications Research, and Ann Arbor VA Center for Clinical Management Research.
2009

Lona Mody launches a major research effort in nursing homes that leads to a better understanding of how to prevent infections and antibiotic-resistant bacteria — and to an Agency for Healthcare Research and Quality collaborative to implement lessons learned in 500 facilities across the U.S.
2010s
2010
UMHS launches the Joint Institute for Translational and Clinical Research, a partnership with Peking University Health Sciences Center. Under the co-leadership of Joseph Kolars, the institute will conduct collaborative research that leverages the strengths of each university to advance global health. Along with a PUHSC co-lead, Internal Medicine faculty head programs in cardiovascular disease (Eugene Chen), liver disease (Chung Owyang), pulmonary disease (Margaret Gyetko), and renal disease (Matthias Kretzler). By sharing data and expertise, researchers begin comparing the populations in areas such as genetics, environmental factors, and the microbiome to discover the role these and other factors play in health and disease.
2010

The Brehm Tower is built, housing the Brehm Center for Diabetes Research. Center researcher, Peter Arvan, will use the infrastructure to implicate protein folding errors in a severe form of early-onset diabetes. Arvan, who will later help launch the medical school’s Protein Folding Diseases Initiative, identifies Mutant Insulin\gene-induced Diabetes of Youth (MIDY), where one mutant insulin gene blocks the product of the remaining normal insulin gene. He finds that this is one disease within a more general class of illnesses affecting a poorly understood intracellular organelle called the endoplasmic reticulum.
2010

Research by Caroline Blaum demonstrates the heterogeneity of the older diabetes population and suggests the need for a more personalized approach to glycemic control for older patients with diabetes. Her work will help shape recommendations by the American Diabetes Association for better tailoring treatment goals to patients’ age, health status, motivation, resources, and complications.
2011

Kenneth Langa becomes associate director of the Health and Retirement Study, a longitudinal survey of 20,000 U.S. adults and U-M’s largest extramurally funded research project. He and colleagues will use the survey to probe issues such as the causes, prevalence, and economic impact of dementia; long-term effects of acute illnesses on the brain and body; and the role of Medicare and Medicaid policies on health outcomes for older adults. One of Langa’s papers on the costs of dementia will receive front-page coverage in The New York Times and inform congressional discussions about funding for dementia care and research.
2011
James Woolliscroft expands Global REACH (Research, Education, and Collaboration for Health) as an institution-wide mechanism for fostering collaborative research and training in global health. Started in 2001 under the initial leadership of David Stern (Gen), followed by Joseph Kolars (GI) in 2009, the program will evolve to support substantial research platforms in Brazil, China, Ethiopia, Ghana, and India, and more than 20 additional formal international relationships. In 2015, UMMS global health researchers will produce 100-plus publications and garner nearly $150 million in extramural grant funding.
2011

Raymond Yung uses animal models to begin unraveling the molecular mechanisms of aging by publishing one of the first studies showing that age-related obesity results in more pro-inflammatory immune cells and proteins compared to diet-induced obesity. He will later show how epigenetic changes, especially DNA methylation and histone acetylation, accumulate during aging and contribute to autoimmunity risk.
2011

Ronald Koenig shows that the type 2 diabetes drug pioglitazone can be used to treat a special form of follicular thyroid cancer, transforming the cancer cells into more differentiated fatlike cells that have lost their malignant character. This discovery stems from Koenig’s earlier finding that this unusual cancer is triggered by the Pax8-PPARgamma fusion protein that inappropriately connects two unrelated transcription factors. Because pioglitazone binds to PPARgamma, Koenig explores its effects in mouse models of follicular thyroid cancer and, based on the results, ushers pioglitazone into clinical trials.
2011

Steroid biochemist Richard Auchus is recruited and establishes a steroid analysis platform within U-M’s metabolomics program. Over the next five years, his team will conduct two phase 1 trials of novel treatments for 21-hydroxylase deficiency, an enzyme defect in cortisol synthesis, which results in excess production of androgens, or male hormones. Their “steroidomics” platform will identify unrecognized steroid hormones in this disease as well as biomarker steroids to monitor disease control. His laboratory will also discover critical biochemical mechanisms of androgen production and drug action in prostate cancer.
2011
The NIH splits its Diabetes Research and Training Centers into two new entities, and U-M is awarded both. The Center for Diabetes Translational Research is directed by Bill Herman, whose research on the epidemiology and economics of diabetes has shown that lifestyle changes can be more effective than medication in reducing risk for and treating type 2 diabetes. The Diabetes Research Center (DRC) is directed by Martin Myers (MEND), a world leader in understanding how the hormone leptin acts on the brain to regulate fertility, calorie expenditure, appetite, and satiety.
2011

Megan Haymart calls providers’ attention to the overtreatment of low-risk thyroid cancer, first examining radioactive iodine and, later, surgery. She shows that variation in hospital characteristics influences treatment decisions more than disease severity, exposing patients to unnecessary risks.
2012

John Ayanian is named the first director of the Institute for Healthcare Policy and Innovation. This campus-wide initiative will grow to include 470 health services researchers — more than 80 from U-M Internal Medicine — whose work aims to inform policymakers about how to improve the quality, safety, equity, and affordability of healthcare. IHPI research will help shape payer policy, healthcare guidelines, and international campaigns on appropriate health care utilization.
2012
U-M contributes to the personalized treatment of COPD through SPIROMICS, the NIH’s Subpopulations and Intermediate Outcomes in COPD Study. Meilan Han and Fernando Martinez, along with imaging collaborators, publish a new radiographic biomarker called the parametric response map that can measure airway thickness via CT scans to subtype COPD patients by disease type, severity, location, and distribution. Pulmonary researchers, led by Jeffrey Curtis, also identify specific lymphocyte populations and cytokines involved in COPD progression. This research motivates a landmark clinical trial demonstrating the efficacy of azithromycin to reduce COPD exacerbations.
2012

Eugene Chen establishes the Center for Advanced Models for Translational Sciences and Therapeutics to develop large animal models to accelerate bench-to-bedside biomedical research and drug development. The center is among the first in the world to establish rabbit embryonic stem cell lines, to generate cloned rabbits, and to produce knockout and knock-in pigs and rabbits.
2012

Steven Katz, director of the Cancer Surveillance and Outcomes Research Team, receives a National Cancer Institute project award to research the challenges of individualizing treatments for patients with early-stage breast cancer. Findings have highlighted the role of personalized communication and decision-making on patient experiences and outcomes. The research has informed best practices and has markedly contributed to methodology in the growing field of oncology population and implementation sciences.
2013

Pavan Reddy (Hem/Onc) demonstrates two promising approaches for prevention and treatment of graft versus host disease, as well as the mechanisms behind them. He shows that histone deacetylase inhibition can affect target tissues and cut the incidence of GVHD, and demonstrates the potential for alpha-1-antitrypsin to treat steroid-refractory GVHD. He also implicates the microRNA miR-142 and its targets in GVHD, identifying potential future drug targets.
2013
The medical school launches the Host Microbiome Initiative with broad Department of Internal Medicine involvement, including leadership from microbiologists Vincent Young and Thomas Schmidt. The initiative provides infrastructure that supports systems-level research on how the microbial communities on and in our bodies impact health and disease. Topics range from unraveling the therapeutic mechanisms of fecal transplant for treating recurrent C. difficile to studying the role of the gut microbiota in the pathogenesis of inflammatory bowel disease, lupus, multiple sclerosis, food allergy, obesity, and colon cancer. Gary Huffnagle and others in PCCM will use the infrastructure to demonstrate the importance of gut and lung microbiota in the pathogenesis of inflammatory lung diseases, including asthma, COPD, and ARDS.
2013

Joseph Holoshitz advances our understanding of the genetic and molecular basis of rheumatoid arthritis (RA) by showing how variants of the human leukocyte antigen cause RA. His findings show that a subset of genes that code for “shared epitope” proteins lead to joint inflammation and bone erosion not by causing the body to mistakenly identify its own tissues as foreign, but by directly activating inflammation-causing and bone-destroying cells. He will also show that compounds targeting this pathway demonstrate antiarthritic and anti-inflammatory effects, and may provide a joint targeted alternative to the whole body immune suppression from current biologics.
2014
The FDA approves a U-M-invented drug for Gaucher disease, a genetic disease in which a deficiency of the enzyme glucocerebrosidase causes fatty substances to accumulate in cells and organs. Co-developed over more than a quarter-century by James Shayman, the new drug, eliglustat tartrate (Cerdelga), is an oral glycolipid synthesis inhibitor that provides an alternative to intravenous enzyme replacement. As the first stand-alone oral agent for this lysosomal storage disease, eliglustat is now used worldwide and may become the standard of care for Gaucher type 1.
2014

Under the direction of Rajiv Saran, the United States Renal Data System coordinating center is again awarded to the U-M Kidney Epidemiology and Cost Center — which previously received a nearly $18 million grant from the Centers for Medicare and Medicaid Services. These awards affirm U-M’s importance in national kidney disease monitoring, quality improvement and research.
2014
Colin Cooke and Jack Iwashyna publish a quality framework for improving sepsis care based on their research. They also develop the concept of “survivorship” after acute illness, identifying long-term physical and neurocognitive impairments in sepsis patients. The changes they advocate — including early identification of sepsis, incentivizing best practices, and developing new models of care — have the potential to meaningfully improve outcomes for sepsis patients worldwide.
2014
U-M is the only institution in the country to receive both basic and clinical NIH Autoimmunity Center of Excellence grants. The basic science grant, under Bruce Richardson (Rheum), funds state-of-the-art genomic and epigenomic approaches to unravel the mechanisms causing lupus, identify new therapeutic targets, and test a novel biomarker of disease progression. The clinical grant, under David Fox, supports mechanistic studies of treatments for multiple sclerosis and scleroderma, and explorations of how clustered autoimmune diseases are initiated.
2014

With colleagues in pharmacology and pharmacy, Dinesh Khanna leads a group developing new compounds to fight fibrosis in scleroderma, a disease with limited treatment options. The compounds target the genetic switch that controls the formation of myofibroblasts — the cells that produce too much collagen, leading to skin thickening and organ damage. The team shows biological effects in cell lines and animal models, and will move on to compound optimization in preparation for human drug-testing.
2015

After helping to clarify the beneficial role of naturally occurring gases such as nitric oxide and carbon monoxide in the cardiovascular system, David Pinsky shows that the enzyme CD39 can prevent atherosclerosis in mice. A membrane-bound enzyme that lines human blood vessels, CD39 expression is greatest where blood flow is smooth and reduced at bend and branch points where blood flow is turbulent — which is also where plaque naturally tends to accumulate. His group is hopeful that the protective role of CD39 will have implications for the management of cardiovascular disease.
2015

David Markovitz publishes the first demonstration that two functions of a lectin (sugar-binding protein) can be separated through targeted molecular engineering. The resultant molecule, which is derived from a compound found in bananas, could become a clinically effective broad-spectrum antiviral agent, as it is highly active against influenza, MERS, SARS, Ebola virus, HIV, and hepatitis C virus. Engineering of lectins also holds promise for helping to unravel the mysteries of the Sugar Code, the mechanism by which complex sugars transmit information in the body.
Sources: Department of Internal Medicine division histories, division chief and faculty interviews and the
U-M Medical School historical timeline.



