

The NIH T32 Clinical Research Training Program (DK062708), established in 2003, is co-directed by Dr. Peter D. Higgins and Dr. Sameer D. Saini. Dr. Higgins and Dr. Saini are supported by our multidisciplinary core faculty that includes expert biostatisticians, epidemiologists, behavioral economists, policy-makers, and translational, clinical, and outcomes researchers.
Our core faculty come from not only the Division of Gastroenterology and Hepatology, but also from the Division of General Medicine and the School of Public Health. The majority of our faculty are federally funded for their ongoing research and offer a broad spectrum of research expertise with multiple levels of established collaboration and many years of experience mentoring trainees.
Program Goal
The goal of our four-year program is to train and develop future independent investigators and leaders in gastroenterology and hepatology clinical and health services research by providing selected fellows with:
- Core methodological skills in the design and execution of clinical research
- A structured, mentored research experience under the guidance of our world-class core faculty
With this training, our T32 fellows leave fellowship poised to compete for tenure-track faculty positions and secure external funding for further career development, ultimately leading to independent research careers.
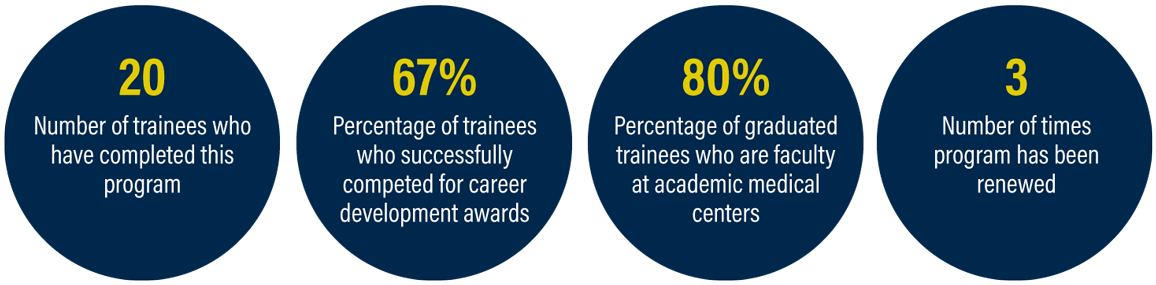
Program Objectives
By the end of the program, trainees will have completed the below objectives.
- Master's degree focused on the design and execution of clinical research at the U-M Institute for Healthcare Policy and Innovation National Clinician Scholars Program (Year 2)
- Focused GI clinical research seminar series, a comprehensive clinical epidemiology/health care policy seminar series, and ongoing seminars and workshops (Years 2–4)
- Multiple research projects (designed, executed, and published) under the guidance of a team of mentors, including gastroenterologists, hepatologists, and methodologists (Years 2–4)
- Career development award application or other research grant application (Year 4)
More About Our Program
Each trainee will focus on a specific topic and complete three interrelated projects:
- a systematic review +/- meta-analysis
- a secondary dataset analysis
- design of a prospective study
These projects provide the foundation and preliminary data for a career development award application.
Our training program is greatly enhanced by the rich, collaborative environment at Michigan Medicine, with vast resources, centers, and research institutes, which include:
- Michigan Institute of Clinical and Health Research (CTSA-supported)
- Institute for Healthcare Policy and Innovation
- VA Center for Clinical Management Research (VA HSR&D Center of Innovation)
Leadership Team
Dr. Peter D. Higgins – Co-Director
Dr. Higgins, an internationally recognized leader in inflammatory bowel disease (IBD). Dr. Higgins is a Professor of Gastroenterology in the Department of Internal Medicine at the University of Michigan and Director of the Inflammatory Bowel Disease Program at the University of Michigan. He also previously served as Associate Director of the Training Program in GI Epidemiology and is himself a graduate of this Training Program.
Dr. Higgins’ research focuses on clinical trials in IBD, translational research in measuring intestinal fibrosis in Crohn’s disease, development of anti-fibrotic and anti-inflammatory therapies, and the use of machine learning for predicting response to IBD therapies. He previously led the Crohn’s and Colitis Foundation of America (CCFA) Clinical Research Alliance and currently chairs the AGA IMIBD section, leads the IBD Working Group at the University of Michigan, and is the National Scientific Advisory Committee (NSAC) chair-elect for the Crohn’s and Colitis Foundation. He has published over 215 research papers and has received multiple NIH grants and foundation awards for his research in IBD. His current funding portfolio includes two R01s (R01DK118154 and R01DK125687) and over 50 active clinical studies in IBD. Dr. Higgins has served as the Associate Editor for the American Journal of Gastroenterology and Inflammatory Bowel Diseases. His research has impacted policy related to IBD through his work on the AGA IBD Quality Measures Committee and the development of national guidelines for IBD. He has also received many honors and awards, including the AGA IBD Clinical Research Excellence Award.
Dr. Higgins has trained more than 30 fellows and early career faculty over the course of his career, several of whom have gone on to become independent investigators, including Dr. Akbar Waljee and Dr. Ryan Stidham. Notably, as an MD-PhD-MSc, he brings expertise in not only outcomes research, but also translational research and clinical trials. Dr. Higgins is the current chair of the Division’s Research Advisory Council, which oversees research progress and academic development of fellows and early career faculty.
Dr. Sameer D. Saini – Co-Director
Dr. Saini is an internationally-recognized leader in GI health services research. A former graduate of this Training Program, he is now a Professor of Gastroenterology in the Department of Internal Medicine at the University of Michigan. He is also the Director of the VA Ann Arbor Center for Clinical Management Research (CCMR), a VA HSR&D Center of Innovation with nearly $20 million of annual funding which comprises more than 40 investigators from diverse disciplines across Departments and Schools as well as over 150 staff. Additionally, he serves as Director of the Gastroenterology Early Career Faculty Health Services Research Mentoring Program. He is a standing member of the VA HSR&D study section for Career Development Awards and a former member of the ACG Research Committee.
Dr. Saini’s research focuses on appropriate use of resource-limited medical procedures, such as colonoscopy and upper endoscopy, development of “next generation” performance measures, and development and testing of multi-level interventions to reduce the use of low-value care. His research has been continuously federally funded since 2010. In addition to the VA HSR&D Center of Innovation Program Grant, his current funding as Principal Investigator (PI) or Co-PI includes two VA HSR&D Merit Awards and a VA HSR&D Consortium of Research. Altogether, his current funding as PI exceeds $10 million. He works closely with national operational leaders on topics related to specialty care access and quality. Dr. Saini’s mentees include Dr. Megan Adams (Core Faculty on this Training Program who serves as an “ombudsman” for trainees) and Dr. Jacob Kurlander (an independently-funded investigator with VA QUERI and NIH K23 funding). Dr. Saini currently serves on the American College of Physicians (ACP) Performance Measures Committee and is former Lead of the Colorectal Cancer Screening Workgroup for the AGA Quality Measures Committee. He also serves as Co-Editor of the “Red Section” of the American Journal of Gastroenterology.
Core Faculty
Megan A. Adams, MD, JD, MSc, Assistant Professor of Medicine, is a 2015 graduate of the T32 Training Program. She is a Research Investigator with the VA Ann Arbor HSR&D Center for Clinical Management Research and a member of the University of Michigan Institute for Healthcare Policy and Innovation. As a general gastroenterologist, attorney, and health services researcher, her work focuses on optimizing specialty care access and delivery and promoting high-value use of healthcare resources. Dr. Adams is the recipient of an American College of Gastroenterology Junior Faculty Development Grant (2018-2021) focused on promoting high-value use of GI endoscopic sedation and received the 2019 ACG Governors Award for Excellence in Clinical Research. She currently is independently funded by VA/HSR&D for work focused on optimizing specialty virtual care delivery and understanding the impacts of COVID-19 and VA community care expansion on specialty care delivery within the VA. Her research has been published in journals including JAMA, JAMA Internal Medicine, and Gastroenterology, and she also frequently contributes invited articles on important clinical practice and health law topics impacting frontline gastroenterology policy and practice. Dr. Adams has been an active participant in national efforts related to quality measurement and performance improvement, serving previously as Chair of AGA’s Quality Committee (2017-20) and Co-Chair of the GI Workgroup of the NQF-facilitated Core Quality Measures Collaborative (CQMC) (2018-21). She currently serves as Chair of the AGA Ethics and Audit Committees and is incoming Editor-in-Chief of GI & Hepatology News (2021-26).
William D. Chey, MD, Chief of the GI and Hepatology Division and Professor of Medicine, is a renowned expert in functional gastrointestinal disorders (FGIDs) who has a long track record of research addressing the pathophysiology, diagnosis, and treatment of these highly prevalent and debilitating disorders. His work has been well-funded through multiple industry and foundation grants, including several that are currently active. He holds leadership positions at Michigan Medicine and in national organizations. He is a member of the Board of Trustees of the American College of Gastroenterology and the Council of the American Neurogastroenterology and Motility Society. He was an integral part of the Rome III and Rome IV processes, which were responsible for developing the diagnostic criteria for the FGIDs. He is co-inventor of the digital health platforms “My GI Health” and “My Nutrition Health”, as well as a glove-based manometry device called “Digital Manometry”. He was Co-Editor-In-Chief of the American Journal of Gastroenterology from 2010-2015. Dr. Chey supervises a rapidly growing program of dietary and behavioral interventions for FGIDs at the University of Michigan. He is a member of the League of Research Excellence at the University of Michigan. He pioneered the concept of teaching patients how to prepare meals that would decrease their GI symptoms through establishment of a “kitchen” and cooking classes. In 2019, he was honored with the Dean’s Award for Innovation and Commercialization. He has also received the Distinguished Clinician Award from the American Gastroenterological Association. His expertise will be invaluable for trainees with clinical research projects related to FGIDs.
Raymond De Vries, PhD, is a Professor emeritus in the Department of Learning Health Sciences, the Department of Obstetrics and Gynecology, and the Department of Sociology. He was Associate Director of the Center for Bioethics and Social Sciences in Medicine (CBSSM) 2010-2020. He is a medical sociologist with broad experience in qualitative and deliberative methods and his NIH-funded work examines societal and ethical issues in medicine in several clinical domains. He serves as a methodological expert for trainees who use qualitative and mixed methods.
Robert J. Fontana, MD, Professor of Medicine and Medical Director of Liver Transplantation, is an internationally recognized expert in acute liver failure, drug-induced liver injury, and viral hepatitis. He has published over 250 papers on a variety of topics and has been a co-author of the AASLD Hepatitis C Guidance. He is the current co-chair of the U01-funded Drug-Induced Liver Injury (DILI) Network (U01DK065184) Steering Committee that was initiated in 2013. He has led analyses exploring the development of chronic liver injury from drugs, as well as genetic polymorphisms associated with individual drugs. As a national/international expert in DILI research, he has served as chair of the AASLD Hepatotoxicity Special Interest Group and has been a participant and co-chair of the AASLD-FDA-Pharma meeting on DILI for the past 15 years. He is also a co-PI on the US Acute Liver Failure Study Group, which has been working to improve our knowledge of the etiology, outcomes, and treatment of acute liver failure. Dr. Fontana also has served as the lead investigator on long-term outcomes in acute liver failure and has been the PI on various ancillary studies involving hepatitis A, hepatitis E, ischemia, and transplantation for acute liver failure. He has also participated in various clinical trials and is the current PI of the Methacetin Breath Test Study, which is attempting to identify a noninvasive predictor of outcomes in acute liver failure. Dr. Fontana is also a member of the Hepatitis B Research Network and serves as the Chief of the Publications Committee. Dr. Fontana is a current member of the AASLD’s Clinical Research Committee and has served on the AGA Research Committee. He has also been a grant reviewer for the NIH and FDA study sections for the past 20 years. He also has extensive experience using large datasets to evaluate outcomes related to liver transplantation. Dr. Fontana’s expertise will be invaluable to trainees with an interest in complications of liver disease and the use of large datasets to assess outcomes in liver disease patients.
Rodney Hayward, MD, Professor of Medicine in the Division of General Internal Medicine, is Director of the IHPI Clinician Scholars Program and the Director of University of Michigan's MSc program in Health & Health Care Research and an NIDDK-funded Methods Core. His research focuses on quality improvement and application of tailored clinical guidelines to improve personalized patient care. Dr. Hayward brings a wealth of experience in the organization and administration of training programs. He also brings significant leadership experience in mentoring and facilitating the performance of outcomes and health services research by post-doctoral trainees.Along with members of the leadership team, Dr. Hayward serves on the selection committee for the training program.
H. Myra Kim, ScD, is Adjunct Professor of Biostatistics in the School of Public Health, Research Scientist in the Consulting for Statistics, Computing, and Analytics Research (CSCAR), and Core Faculty in the IHPI Clinician Scholars Program. Dr. Kim is currently a co-investigator and principal statistical investigator on several NIH, Department of Defense, and Veterans Health Administration sponsored multi-national and multi-center randomized clinical trials, cluster randomized trials, and observational cohort studies. Her work has focused on applying advanced statistical methods to assess health outcomes, particularly in collaboration with investigators conducting clinical trials and analyses of large-scale observational datasets. Dr. Kim collaborates on the study design and statistical data analysis plan for faculty research projects in the Department of Internal Medicine and the VA Center for Clinical Management Research. She also mentors our trainees on statistical planning/data analysis for their research projects.
Anna S. F. Lok, MD is the Dame Sheila Sherlock Distinguished University Professor in Internal Medicine and Hepatology, Alice Lohrman Andrews Research Professor in Hepatology, and Director of Clinical Hepatology in the Department of Internal Medicine. Dr. Lok also serves as Assistant Dean for Clinical Research in the University of Michigan Medical School (where she leads the clinical trials enterprise for the medical school), and is the former President of the American Association for the Study of Liver Diseases (AASLD). Dr. Lok is an internationally recognized physician scientist, hepatologist, leader, and mentor. Her research includes bedside clinical, T1, and T2 studies and has published more than 550 articles. Dr. Lok was recognized as one of the top 1% most cited scientists in the world during the period of 2002–2012. Her research has been supported by federal grants from the National Institutes of Health (NIH) and the Veterans Health Administration (VA). Currently, Dr. Lok is Chair of the Steering Committee on the Hepatitis B Clinical Research Network (U01DK082863), a disease in which she is arguably the world’s foremost expert, and is also Chair of the Steering Committee of the Translational Liver Cancer Network (U01CA230669). Dr. Lok has also served as an author and working group member for AASLD and World Health Organization (WHO) guidelines on HBV and HCV. Dr. Lok has trained more than 50 fellows and early career faculty over the course of her career, many of whom have gone on to become independent investigators and leaders in hepatology. In recognition of her accomplishments as a scientist, mentor, and leader, Dr. Lok has received numerous awards including the Distinguished Women Scientist Award from the American Gastroenterological Association (AGA) in 2008, the Distinguished Service Award from the AASLD in 2011, the Distinguished Mentor Award from MICHR in 2012, and an Inspirational Physician Award from the American Medical Association in 2014. In 2016, she was awarded the AGA’s William Beaumont Prize in Gastroenterology, which recognizes an individual who has made a unique, outstanding contribution of major importance to the field. In 2017, Dr. Lok received a Gold Medal from the Canadian Association for the Study of the Liver and an Honorary Doctor of Science degree from her alma mater, the University of Hong Kong. In 2018, she received an International Recognition Award from the European Association for the Study of the Liver. In 2019, Dr. Lok received a Distinguished University Professorship from the University of Michigan. She served as an Associate Editor of Hepatology from 2001-2006, Co-Editor of Journal of Viral Hepatitis from 2007-2009, and Senior Associate Editor of Gastroenterology from 2011-2012.
Joel H. Rubenstein, MD, MSc, Professor of Medicine, is the Director of the Barrett’s Esophageal Disorders Program at University of Michigan and a Research Scientist at the VA Center for Clinical Management Research. Dr. Rubenstein’s primary research focus is on risk factors for Barrett’s esophagus and esophageal adenocarcinoma, and developing and testing efficient strategies for decreasing the burden of esophageal adenocarcinoma. He uses epidemiologic, outcomes, and translational tools. He also has a focus on appropriate utilization of healthcare resources in gastroenterology. Dr. Rubenstein is a former graduate of our Training Program. He provides content and methodological expertise for trainees with an interest in esophageal diseases and cancer prevention.
Elliot Tapper, MD, Associate Professor of Medicine, is Director of the Michigan Cirrhosis Program. His primary research interest is cirrhosis outcomes, focusing on clinical trials aimed at improving quality of life, large data / claims analysis, cost-effectiveness modeling, and quality improvement. He has mentored multiple T32 fellows in projects that have included meta-analyses, claims data analyses, and prospective cohort studies. Dr. Tapper’s research is supported by an NIH K23 grant and a U01 grant. He is the recipient of the 2021 AGA Distinguished Young Investigator Award – Clinical. He has published more than 130 papers.
Sandeep Vijan, MD, MS, Professor of Medicine in the Division of General Internal Medicine, is a Research Scientist at the VA Center for Clinical Management Research and Director of Quality Analytics at the University of Michigan. Dr. Vijan conducts health services and policy research on individualizing decisions around treatment of chronic diseases using statistical modeling methods, including econometrics and simulation methodology. His research has been funded by NIH and VA. He mentors trainees in simulation modeling, quality measurement, and economic analysis. Additionally, in his role as Director of Quality Analytics for the health system, he facilitates access to data from the University of Michigan Hospitals for secondary data analysis.
Akbar K. Waljee, MD, MSc, is a Professor of Medicine. Dr. Waljee serves as the Associate Director of the Data and Methods Hub and the Director of the Michigan Integrated Center for Health Analytics and Medical Prediction (MiCHAMP) both at the UM Institute for Healthcare Policy & Innovation (IHPI). He also serves as the Director of the VA CCMR Prediction Modeling Unit (PMU) and, clinically, he is the Director of the Inflammatory Bowel Disease Clinic at the VA Ann Arbor Healthcare System. Dr. Waljee’s work is at the forefront of using machine learning and deep learning techniques to improve healthcare access, quality, and efficiency (high-value care) in resource constrained settings. He uses novel machine learning techniques to implement decision support systems and tools that facilitate more personalized care for disease management and healthcare utilization to ultimately deliver efficient, effective, and equitable therapy for chronic diseases. To test and advance these principles, he built operational programs that are guiding—and improving—patient care in costly gastroenterology and liver disorders in under-resourced settings both domestically and abroad. Dr. Waljee was awarded the MICHR Distinguished Mentoring Award in 2019.
Brian Zikmund-Fisher, PhD, is Professor of Health Behavior and Health Education in the School of Public Health and a Research Professor of Internal Medicine. He is also Associate Director of the Center for Bioethics and Social Sciences in Medicine (CBSSM) and the current Editor-in-Chief of the journals Medical Decision Making and MDM Policy & Practice. Dr. Zikmund-Fisher uses his interdisciplinary background in decision psychology and behavioral economics to study factors that affect individual decision making about a variety of health and medical issues. His research in health communications focuses on making risk statistics and other types of quantitative health information meaningful and useful for decision making by patients and the public. An expert in survey design, Dr. Zikmund-Fisher has assisted numerous trainees from our Training Program, providing methodological guidance and support related to survey research, development of patient education and risk communication materials, and medical decision-making.
Former Trainee Testimonials
Eric Shah, MD, MBA, FACG – 2018 Graduate
“I am grateful for the Michigan T32 GI Epidemiology Training Program, which supported my training in health services research and medical innovation during fellowship and enabled me to obtain a tenure-track faculty position at Dartmouth-Hitchcock Medical Center. The T32 provided me with the foundational didactic knowledge and skillset as well as time to generate preliminary data that allowed me to successfully compete for a career development award supported by the AGA Research Foundation. Most importantly, the T32 provided the mentored opportunity to learn a team-based, multidisciplinary approach to academic research spanning several institutes at the University of Michigan and Michigan Medicine both inside and outside of the Division of Gastroenterology and Hepatology."
Megan A. Adams, MD, JD, MS – 2015 Graduate
“The T32 supported my training in health services research as a fellow, allowed me to secure a tenure-track faculty position at a world-class research university, and provided me with the background and preliminary data to successfully compete for a career development award through ACG and more recently funding through the VA. It also provided me with mentoring from multidisciplinary faculty inside and outside our Division of Gastroenterology and Hepatology. As a result, I am now well on my way to an independently-funded research career.”
Jessica L. Mellinger, MD, MS – 2014 Graduate
“The T32 supported my training in health services research as a fellow, allowed me to secure a tenure-track faculty position at world-class research University. It also provided me with the training, mentoring and preliminary data to successfully compete for a career development award through AASLD and later NIH. The clinical and research support I receive together with the protected time allowed me to establish myself as a nationally recognized expert at an early stage in my career. I was invited to co-author the AASLD guidelines on alcoholic liver diseases and have since been invited to speak at national as well as international meetings, and to be a visiting professor at other academic medical centers. I have also started giving back through mentoring of fellows and early career faculty at the University of Michigan as well as other hepatology programs.”
How to Apply
Our Clinical T32 trainees are selected from the pool of GI fellowship applicants based on a competitive application process. If you are interested in applying, please indicate your interest on your ERAS application, and we will reach out to you ahead of your interview day to ensure that you meet with faculty with aligned interests.
Contact Us
Please email [email protected] if you have questions or would like more information about our program.



