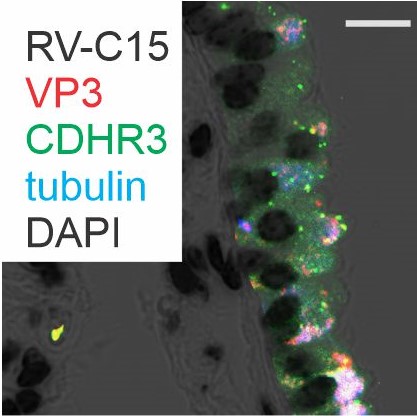
The Division of Pediatric Pulmonology directs investigator-initiated basic and clinical research programs related to asthma, cystic fibrosis and bronchopulmonary dysplasia. Faculty research is funded by the National Institutes of Health and Cystic Fibrosis Foundation. Areas of study include:
Asthma
- Viral-induced exacerbations of asthma and other chronic airways diseases
- Contribution of early-life respiratory viral infections in the development of asthma
- Matricellular proteins in the pathogenesis of asthma and bronchopulmonary dysplasia
- Interactive effects of diesel exhaust and respiratory viral infection on asthmatic children
- Promoting activity among children with asthma in Detroit
Cystic Fibrosis
- Role of viral and bacterial co-infection in the pathogenesis of cystic fibrosis lung disease
- Influence of the airway microbiome on nontuberculous mycobacterial infection in cystic fibrosis
- Interventions to improve adherence in children with cystic fibrosis
- Studies to improve the cystic fibrosis newborn screening parental notification process
Bronchopulmonary dysplasia
- Matricellular proteins in the pathogenesis of asthma and bronchopulmonary dysplasia
- Damage-associated molecular patterns and priming of the immune system in bronchopulmonary dysplasia
- Role of adipose-tissue macrophages in the pathogenesis of obesity-associated diseases such as metabolic syndrome and diabetes
Faculty Research Interests